Long chain saturated and unsaturated fatty acids exert opposing effects on viability and function of GLP-1-producing cells: Mechanisms of lipotoxicity
- PMID: 28520810
- PMCID: PMC5433723
- DOI: 10.1371/journal.pone.0177605
Long chain saturated and unsaturated fatty acids exert opposing effects on viability and function of GLP-1-producing cells: Mechanisms of lipotoxicity
Abstract
Background and aim: Fatty acids acutely stimulate GLP-1 secretion from L-cells in vivo. However, a high fat diet has been shown to reduce the density of L-cells in the mouse intestine and a positive correlation has been indicated between L-cell number and GLP-1 secretion. Thus, the mechanism of fatty acid-stimulated GLP-1 secretion, potential effects of long-term exposure to elevated levels of different fatty acid species, and underlying mechanisms are not fully understood. In the present study, we sought to determine how long-term exposure to saturated (16:0) and unsaturated (18:1) fatty acids, by direct effects on GLP-1-producing cells, alter function and viability, and the underlying mechanisms.
Methods: GLP-1-secreting GLUTag cells were cultured in the presence/absence of saturated (16:0) and unsaturated (18:1) fatty acids (0.125 mM for 48 h, followed by analyses of viability and apoptosis, as well as involvement of fatty acid oxidation, free fatty acid receptors (FFAR1) and ceramide synthesis. In addition, effects on the expression of proglucagon, prohormone convertase 1/3 (PC1/3), free fatty acid receptors (FFAR1, FFAR3), sodium glucose co-transporter (SGLT) and subsequent secretory response were determined.
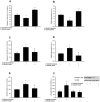
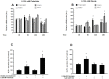
Results: Saturated (16:0) and unsaturated (18:1) fatty acids exerted opposing effects on the induction of apoptosis (1.4-fold increase in DNA fragmentation by palmitate and a 0.5-fold reduction by oleate; p<0.01). Palmitate-induced apoptosis was associated with increased ceramide content and co-incubation with Fumonisin B1 abolished this lipo apoptosis. Oleate, on the other hand, reduced ceramide content, and-unlike palmitate-upregulated FFAR1 and FFAR3, evoking a 2-fold increase in FFAR1-mediated GLP-1 secretion following acute exposure to 0.125 mmol/L palmitate; (p<0.05).
Conclusion/interpretation: Saturated (16:0), but not unsaturated (18:1), fatty acids induce ceramide-mediated apoptosis of GLP-1-producing cells. Further, unsaturated fatty acids confer lipoprotection, enhancing viability and function of GLP-1-secreting cells. These data provide potential mechanistic insight contributing to reduced L-cell mass following a high fat diet and differential effects of saturated and unsaturated fatty acids on GLP-1 secretion in vivo.
Conflict of interest statement
Figures

Similar articles
-
Oleate increase neutral lipid accumulation, cellular respiration and rescues palmitate-exposed GLP-1 secreting cells by reducing ceramide-induced ROS.Biochimie. 2019 Apr;159:23-35. doi: 10.1016/j.biochi.2018.11.017. Epub 2018 Dec 1. Biochimie. 2019. PMID: 30513370
-
Molecular mechanisms of lipoapoptosis and metformin protection in GLP-1 secreting cells.Biochem Biophys Res Commun. 2012 Oct 12;427(1):91-5. doi: 10.1016/j.bbrc.2012.09.010. Epub 2012 Sep 11. Biochem Biophys Res Commun. 2012. PMID: 22982676
-
Glucagon-like peptide-1 production in the GLUTag cell line is impaired by free fatty acids via endoplasmic reticulum stress.Metabolism. 2014 Jun;63(6):800-11. doi: 10.1016/j.metabol.2014.02.012. Epub 2014 Feb 25. Metabolism. 2014. PMID: 24680601
-
The cytoprotective actions of long-chain mono-unsaturated fatty acids in pancreatic beta-cells.Biochem Soc Trans. 2008 Oct;36(Pt 5):905-8. doi: 10.1042/BST0360905. Biochem Soc Trans. 2008. PMID: 18793159 Review.
-
Unsaturated fatty acids as cytoprotective agents in the pancreatic beta-cell.Prostaglandins Leukot Essent Fatty Acids. 2010 Apr-Jun;82(4-6):231-6. doi: 10.1016/j.plefa.2010.02.018. Epub 2010 Mar 4. Prostaglandins Leukot Essent Fatty Acids. 2010. PMID: 20206490 Review.
Cited by
-
Restoration of GLP-1 secretion by Berberine is associated with protection of colon enterocytes from mitochondrial overheating in diet-induced obese mice.Nutr Diabetes. 2018 Sep 24;8(1):53. doi: 10.1038/s41387-018-0061-x. Nutr Diabetes. 2018. PMID: 30250193 Free PMC article.
-
Therapeutic Potential of Infrared and Related Light Therapies in Metabolic Diseases.Int J Mol Sci. 2025 May 27;26(11):5134. doi: 10.3390/ijms26115134. Int J Mol Sci. 2025. PMID: 40507946 Free PMC article. Review.
-
G protein-coupled receptors as potential targets for nonalcoholic fatty liver disease treatment.World J Gastroenterol. 2021 Feb 28;27(8):677-691. doi: 10.3748/wjg.v27.i8.677. World J Gastroenterol. 2021. PMID: 33716447 Free PMC article. Review.
-
The Impact of the Mediterranean Diet on Telomere Biology: Implications for Disease Management-A Narrative Review.Nutrients. 2024 Aug 2;16(15):2525. doi: 10.3390/nu16152525. Nutrients. 2024. PMID: 39125404 Free PMC article. Review.
-
Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing.Nutrients. 2021 Jun 6;13(6):1951. doi: 10.3390/nu13061951. Nutrients. 2021. PMID: 34204057 Free PMC article. Review.
References
-
- Rask E, Olsson T, Soderberg S, Johnson O, Seckl J, Holst JJ, et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001;24(9):1640–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

