Optochemical Control of Biological Processes in Cells and Animals
- PMID: 28521066
- PMCID: PMC6026863
- DOI: 10.1002/anie.201700171
Optochemical Control of Biological Processes in Cells and Animals
Abstract
Biological processes are naturally regulated with high spatial and temporal control, as is perhaps most evident in metazoan embryogenesis. Chemical tools have been extensively utilized in cell and developmental biology to investigate cellular processes, and conditional control methods have expanded applications of these technologies toward resolving complex biological questions. Light represents an excellent external trigger since it can be controlled with very high spatial and temporal precision. To this end, several optically regulated tools have been developed and applied to living systems. In this review we discuss recent developments of optochemical tools, including small molecules, peptides, proteins, and nucleic acids that can be irreversibly or reversibly controlled through light irradiation, with a focus on applications in cells and animals.
Keywords: caged compounds; chemical biology; optochemical tools; photochemistry; photoswitches.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
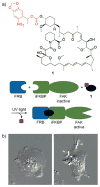

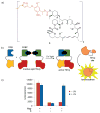
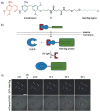




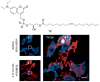
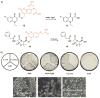




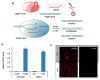














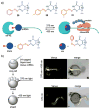


References
-
- Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A. Angew Chem Int Ed. 2012;51:8446–8476. - PubMed
- Angew Chem. 2012;124:8572–8604.
- Mayer G, Heckel A. Angew Chem Int Ed. 2006;45:4900–4921. - PubMed
- Angew Chem. 2006;118:5020–5042.
- Lee HM, Larson DR, Lawrence DS. ACS Chem Biol. 2009;4:409–427. - PMC - PubMed
- Gautier A, Gauron C, Volovitch M, Bensimon D, Jullien L, Vriz S. Nat Chem Biol. 2014;10:533–541. - PubMed
- Klán P, Šolomek T, Bochet CG, Blanc A, Givens R, Rubina M, Popik V, Kostikov A, Wirz J. Chem Rev. 2013;113:119–191. - PMC - PubMed
- Liu Q, Deiters A. Acc Chem Res. 2014;47:45. - PMC - PubMed
- Mart RJ, Allemann RK. Chem Commun. 2016;52:12262–12277. - PubMed
- Ouyang X, Chen JK. Chem Biol. 2010;17:590–606. - PMC - PubMed
-
- Niu J, Johny MB, Dick IE, Inoue T. Biophys J. 2016;111:1132–1140. - PMC - PubMed
- Zhang K, Cui B. Trends Biotechnol. 2015;33:92–100. - PMC - PubMed
- Deisseroth K. Nat Neurosci. 2015;18:1213–1225. - PMC - PubMed
- Weitzman M, Hahn KM. Curr Opin Cell Biol. 2014;30:112–120. - PMC - PubMed
- Pathak GP, Vrana JD, Tucker CL. Biol Cell. 2013;105:59–72. - PMC - PubMed
- Repina N, Rosenbloom A, Mukherjee A, Schaffer DV, Kane RS. Annu Rev Chem Biomol Eg. 2017;8(1):13–39. - PMC - PubMed
-
- Putyrski M, Schultz C. FEBS Lett. 2012;586:2097–2105. - PubMed
- Voss S, Klewer L, Wu YW. Curr Opin Chem Biol. 2015;28:194–201. - PubMed
- Fegan A, White B, Carlson JC, Wagner CR. Chem Rev. 2010;110:3315–3336. - PubMed
- DeRose R, Miyamoto T, Inoue T. Pflugers Archiv: Eur J Physiol. 2013;465:409–417. - PMC - PubMed
-
- Pecot MY, Malhotra V. Cell. 2004;116:99–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

