Endoplasmic reticulum protein ERp46 in prostate adenocarcinoma
- PMID: 28521463
- PMCID: PMC5431273
- DOI: 10.3892/ol.2017.5908
Endoplasmic reticulum protein ERp46 in prostate adenocarcinoma
Abstract
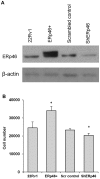
Endoplasmic reticulum (ER) protein ERp46 is a member of the protein disulfide isomerase family of oxidoreductases, which facilitates the reduction of disulfides in proteins and their folding. Accumulation of misfolded proteins has been implicated in cancer. The objectives of the present study were to investigate the role of ERp46 in prostate cancer, its expression and its effects on prostate cancer growth. A tissue microarray with human prostate cancer and normal prostate tissue samples was stained for ERp46 followed by image analysis. Human prostate adenocarcinoma 22Rv1 cells were stably transfected with short hairpin RNA (shRNA) specific for ERp46, a non-effective scrambled control or a plasmid containing full-length human ERp46 cDNA, and cell growth was determined. Subcloned cells were treated with thapsigargin or tunicamycin to induce ER stress and lysates were subjected to western blot analysis for ER stress proteins. Subcutaneous xenografts of parental 22Rv1, ERp46-overexpressing (ERp46+), shERp46 or scrambled control cells were established in male inbred BALB/c nude mice (n=10/group). Tumor growth curves of the xenografts were constructed over a period of 30 days and subsequently the mice were sacrificed and the amount of serum prostate-specific antigen was determined. The results demonstrated increased ERp46 expression levels in prostate cancer tissue samples of Gleason ≥7 compared with normal prostate tissue samples. When ERp46 was stably knocked down using shRNA or overexpressed in prostate carcinoma 22Rv1 cells, tumor growth in vitro and in BALB/c nude mice was inhibited and accelerated, respectively. ERp46 overexpression led to reduced sensitivity to ER stress as indicated by higher half maximal inhibitory concentrations for tunicamycin and thapsigargin in ERp46+ cells. The shERp46 cells lost the ability to upregulate protein disulfide isomerase following tunicamycin-induced ER stress. The present study suggests a role for ERp46 as a therapeutic target in prostate cancer, given its expression profile in human prostate cancer, and its effect on prostate cancer cell growth.
Keywords: endoplasmic reticulum stress; prostate carcinoma; protein disulfide isomerase; thioredoxin.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
