Prion-based memory of heat stress in yeast
- PMID: 28521568
- PMCID: PMC5480388
- DOI: 10.1080/19336896.2017.1328342
Prion-based memory of heat stress in yeast
Abstract
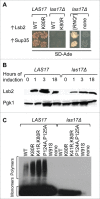
Amyloids and amyloid-based prions are self-perpetuating protein aggregates which can spread by converting a normal protein of the same sequence into a prion form. They are associated with diseases in humans and mammals, and control heritable traits in yeast and other fungi. Some amyloids are implicated in biologically beneficial processes. As prion formation generates reproducible memory of a conformational change, prions can be considered as molecular memory devices. We have demonstrated that in yeast, stress-inducible cytoskeleton-associated protein Lsb2 forms a metastable prion in response to high temperature. This prion promotes conversion of other proteins into prions and can persist in a fraction of cells for a significant number of cell generations after stress, thus maintaining the memory of stress in a population of surviving cells. Acquisition of an amino acid substitution required for Lsb2 to form a prion coincides with acquisition of increased thermotolerance in the evolution of Saccharomyces yeast. Thus the ability to form an Lsb2 prion in response to stress coincides with yeast adaptation to growth at higher temperatures. These findings intimately connect prion formation to the cellular response to environmental stresses.
Keywords: Lsb1; Lsb2; Sup35; actin; amyloid; heat shock; prion; ubiquitin; yeast (Saccharomyces cerevisiae).
Figures

Comment on
- Extra View to: Chernova TA, Kiktev DA, Romanyuk AV, Shanks JR, Laur O, Ali M, Ghosh A, Kim D, Yang Z, Mang M, et al. Yeast short-lived actin-associated protein forms a metastable prion in response to thermal stress. Cell Rep 2017; 18(3):751–761; PMID:28099852; https://doi.org/10.1016/j.celrep.2016.12.082
Similar articles
-
Yeast Short-Lived Actin-Associated Protein Forms a Metastable Prion in Response to Thermal Stress.Cell Rep. 2017 Jan 17;18(3):751-761. doi: 10.1016/j.celrep.2016.12.082. Cell Rep. 2017. PMID: 28099852 Free PMC article.
-
Stress-dependent proteolytic processing of the actin assembly protein Lsb1 modulates a yeast prion.J Biol Chem. 2014 Oct 3;289(40):27625-39. doi: 10.1074/jbc.M114.582429. Epub 2014 Aug 20. J Biol Chem. 2014. PMID: 25143386 Free PMC article.
-
Prion induction by the short-lived, stress-induced protein Lsb2 is regulated by ubiquitination and association with the actin cytoskeleton.Mol Cell. 2011 Jul 22;43(2):242-52. doi: 10.1016/j.molcel.2011.07.001. Mol Cell. 2011. PMID: 21777813 Free PMC article.
-
Physiological and environmental control of yeast prions.FEMS Microbiol Rev. 2014 Mar;38(2):326-44. doi: 10.1111/1574-6976.12053. Epub 2013 Dec 4. FEMS Microbiol Rev. 2014. PMID: 24236638 Free PMC article. Review.
-
The relationship of prions and translation.Wiley Interdiscip Rev RNA. 2010 Jul-Aug;1(1):81-9. doi: 10.1002/wrna.8. Wiley Interdiscip Rev RNA. 2010. PMID: 21339834 Free PMC article. Review.
Cited by
-
Defining the role of the polyasparagine repeat domain of the S. cerevisiae transcription factor Azf1p.PLoS One. 2021 May 21;16(5):e0247285. doi: 10.1371/journal.pone.0247285. eCollection 2021. PLoS One. 2021. PMID: 34019539 Free PMC article.
-
Role of the Cell Asymmetry Apparatus and Ribosome-Associated Chaperones in the Destabilization of a Saccharomyces cerevisiae Prion by Heat Shock.Genetics. 2019 Jul;212(3):757-771. doi: 10.1534/genetics.119.302237. Epub 2019 May 29. Genetics. 2019. PMID: 31142614 Free PMC article.
-
Application of yeast to studying amyloid and prion diseases.Adv Genet. 2020;105:293-380. doi: 10.1016/bs.adgen.2020.01.002. Epub 2020 May 4. Adv Genet. 2020. PMID: 32560789 Free PMC article. Review.
-
Regulation by Different Types of Chaperones of Amyloid Transformation of Proteins Involved in the Development of Neurodegenerative Diseases.Int J Mol Sci. 2022 Mar 2;23(5):2747. doi: 10.3390/ijms23052747. Int J Mol Sci. 2022. PMID: 35269889 Free PMC article. Review.
-
Self-organized computation in the far-from-equilibrium cell.Biophys Rev (Melville). 2022 Dec 27;3(4):041303. doi: 10.1063/5.0103151. eCollection 2022 Dec. Biophys Rev (Melville). 2022. PMID: 38505518 Free PMC article. Review.
References
-
- Chernova TA, Kiktev DA, Romanyuk AV, Shanks JR, Laur O, Ali M, Ghosh A, Kim D, Yang Z, Mang M, et al.. Yeast short-lived actin-associated protein forms a metastable prion in response to thermal stress. Cell Rep 2017; 18(3):751-61; PMID:28099852; https://doi.org/10.1016/j.celrep.2016.12.082 - DOI - PMC - PubMed
-
- Aguzzi A, O'Connor T. Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat Rev Drug Discov 2010; 9(3):237-48; PMID:20190788; https://doi.org/10.1038/nrd3050 - DOI - PubMed
-
- Prusiner SB. Biology and genetics of prions causing neurodegeneration. Ann Rev Genet 2013; 47:601-23; PMID:24274755; https://doi.org/10.1146/annurev-genet-110711-155524 - DOI - PMC - PubMed
-
- Haik S, Brandel JP. Infectious prion diseases in humans: cannibalism, iatrogenicity and zoonoses. Infect Genet Evol 2014; 26:303-12; PMID:24956437; https://doi.org/10.1016/j.meegid.2014.06.010 - DOI - PubMed
-
- Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron 2009; 64(6):783-90. Epub 2010/January/13; PMID:20064386 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
