Role of Ectonucleotidases in Synapse Formation During Brain Development: Physiological and Pathological Implications
- PMID: 28521702
- PMCID: PMC6341498
- DOI: 10.2174/1570159X15666170518151541
Role of Ectonucleotidases in Synapse Formation During Brain Development: Physiological and Pathological Implications
Abstract
Background: Extracellular adenine nucleotides and nucleosides, such as ATP and adenosine, are among the most recently identified and least investigated diffusible signaling factors that contribute to the structural and functional remodeling of the brain, both during embryonic and postnatal development. Their levels in the extracellular milieu are tightly controlled by various ectonucleotidases: ecto-nucleotide pyrophosphatase/phosphodiesterases (E-NPP), alkaline phosphatases (AP), ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases) and ecto-5'- nucleotidase (eN).
Methods: Studies related to the expression patterns of ectonucleotidases and their known features during brain development are reviewed, highlighting involvement of these enzymes in synapse formation and maturation in physiological as well as in pathological states.
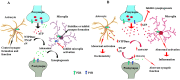
Results: During brain development and in adulthood all ectonucleotidases have diverse expression pattern, cell specific localization and function. NPPs are expressed at early embryonic days, but the expression of NPP3 is reduced and restricted to ependymal area in adult brain. NTPDase2 is dominant ectonucleotidase existing in the progenitor cells as well as main astrocytic NTPDase in the adult brain, while NTPDase3 is fully expressed after third postnatal week, almost exclusively on varicose fibers. Specific brain AP is functionally associated with synapse formation and this enzyme is sufficient for adenosine production during neurite growth and peak of synaptogenesis. eN is transiently associated with synapses during synaptogenesis, however in adult brain it is more glial than neuronal enzyme.
Conclusion: Control of extracellular adenine nucleotide levels by ectonucleotidases are important for understanding the role of purinergic signaling in developing tissues and potential targets in developmental disorders such as autism.
Keywords: Brain development; NPP; NTPDase; TNAP; autism; ecto-5'-nucleotidase; ectonucleotidases; synaptogenesis..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures

Similar articles
-
Trichomonas vaginalis NTPDase and ecto-5'-nucleotidase hydrolyze guanine nucleotides and increase extracellular guanosine levels under serum restriction.Mol Biochem Parasitol. 2016 May;207(1):10-8. doi: 10.1016/j.molbiopara.2016.04.003. Epub 2016 May 2. Mol Biochem Parasitol. 2016. PMID: 27150347
-
Distribution of ectonucleotidases in the rodent brain revisited.Cell Tissue Res. 2008 Nov;334(2):199-217. doi: 10.1007/s00441-008-0681-x. Epub 2008 Oct 9. Cell Tissue Res. 2008. PMID: 18843508
-
Ectonucleotidases and nucleotide/nucleoside transporters as pharmacological targets for neurological disorders.CNS Neurol Disord Drug Targets. 2012 Sep;11(6):739-50. doi: 10.2174/187152712803581092. CNS Neurol Disord Drug Targets. 2012. PMID: 22963442 Review.
-
17β-Estradiol-Induced Synaptic Rearrangements Are Accompanied by Altered Ectonucleotidase Activities in Male Rat Hippocampal Synaptosomes.J Mol Neurosci. 2017 Mar;61(3):412-422. doi: 10.1007/s12031-016-0877-6. Epub 2016 Dec 15. J Mol Neurosci. 2017. PMID: 27981418
-
Therapeutic potentials of ecto-nucleoside triphosphate diphosphohydrolase, ecto-nucleotide pyrophosphatase/phosphodiesterase, ecto-5'-nucleotidase, and alkaline phosphatase inhibitors.Med Res Rev. 2014 Jul;34(4):703-43. doi: 10.1002/med.21302. Epub 2013 Sep 23. Med Res Rev. 2014. PMID: 24115166 Review.
Cited by
-
Functionalized Oxoindolin Hydrazine Carbothioamide Derivatives as Highly Potent Inhibitors of Nucleoside Triphosphate Diphosphohydrolases.Front Pharmacol. 2020 Nov 30;11:585876. doi: 10.3389/fphar.2020.585876. eCollection 2020. Front Pharmacol. 2020. PMID: 33328992 Free PMC article.
-
Enzyme histochemistry: a useful tool for examining the spatial distribution of brain ectonucleotidases in (patho)physiological conditions.Histol Histopathol. 2022 Oct;37(10):919-936. doi: 10.14670/HH-18-471. Epub 2022 May 16. Histol Histopathol. 2022. PMID: 35575291 Review.
-
Guanosine-Based Nucleotides, the Sons of a Lesser God in the Purinergic Signal Scenario of Excitable Tissues.Int J Mol Sci. 2020 Feb 26;21(5):1591. doi: 10.3390/ijms21051591. Int J Mol Sci. 2020. PMID: 32111063 Free PMC article. Review.
-
Synthesis and biological evaluation of sulfamoyl benzamide derivatives as selective inhibitors for h-NTPDases.RSC Adv. 2023 Jul 11;13(30):20909-20915. doi: 10.1039/d3ra03874b. eCollection 2023 Jul 7. RSC Adv. 2023. PMID: 37441049 Free PMC article.
-
Microglial- and Astrocyte-Specific Expression of Purinergic Signaling Components and Inflammatory Mediators in the Rat Hippocampus During Trimethyltin-Induced Neurodegeneration.ASN Neuro. 2021 Jan-Dec;13:17590914211044882. doi: 10.1177/17590914211044882. ASN Neuro. 2021. PMID: 34569324 Free PMC article.
References
-
- Zimmermann H. Nucleotide signaling in nervous system development. Pflugers Arch. 2006;452(5):573–588. [http://dx.doi.org/ 10.1007/s00424-006-0067-4]. [PMID: 16639549]. - PubMed
-
- Majumder P., Trujillo C.A., Lopes C.G., Resende R.R., Gomes K.N., Yuahasi K.K., Britto L.R., Ulrich H. New insights into purinergic receptor signaling in neuronal differentiation, neuroprotection, and brain disorders. Purinergic Signal. 2007;3(4):317–331. [http://dx.doi.org/10.1007/s11302-007-9074-y]. [PMID: 18404445]. - PMC - PubMed
-
- Burnstock G., Ulrich H. Purinergic signaling in embryonic and stem cell development. Cell. Mol. Life Sci. 2011;68(8):1369–1394. [http://dx.doi.org/10.1007/s00018-010-0614-1]. [PMID: 21222015]. - PMC - PubMed
-
- Burnstock G., Dale N. Purinergic signalling during development and ageing. Purinergic Signal. 2015;11(3):277–305. [http://dx. doi.org/10.1007/s11302-015-9452-9]. [PMID: 25989750]. - PMC - PubMed
-
- Oliveira Á., Illes P., Ulrich H. Purinergic receptors in embryonic and adult neurogenesis. Neuropharmacology. 2016;104:272–281. [http://dx.doi.org/10.1016/j.neuropharm.2015.10.008]. [PMID: 26456352]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

