Dopamine D2 Receptors Dimers: How can we Pharmacologically Target Them?
- PMID: 28521704
- PMCID: PMC5883381
- DOI: 10.2174/1570159X15666170518151127
Dopamine D2 Receptors Dimers: How can we Pharmacologically Target Them?
Abstract
Background: Dopamine D2 and D3 receptors can form homo- and heterodimers and are important targets in Schizophrenia and Parkinson's. Recently, many efforts have been made to pharmacologically target these receptor complexes. This review focuses on various strategies to act specifically on dopamine receptor dimers, that are transiently formed.
Methods: Various binding and functional assays were reviewed to study the properties of bivalent ligands, particularly for the dualsteric compound SB269,652. The dimerization of D2 and D3 receptors were analyzed by using single particle tracking microscopy.
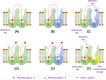
Results: The specific targeting of dopamine D2 and D3 dimers can be achieved with bifunctional ligands, composed of two pharmacophores binding the two orthosteric sites of the dimeric complex. If the target is a homodimer, then the ligand is homobivalent. Instead, if the target is a heterodimer, then the ligand is heterobivalent. However, there is some concern regarding pharmacokinetics and binding properties of such drugs. Recently, a new generation of bitopic compounds with dualsteric properties have been discovered that bind to the orthosteric and the allosteric sites in one monomeric receptor. Regarding dopamine D2 and D3 receptors, a new dualsteric molecule SB269,652 was shown to have selective negative allosteric properties across D2 and D3 homodimers, but it behaves as an orthosteric antagonist on receptor monomer. Targeting dimers is also complicated as they are transiently formed with varying monomer/dimer ratio. Furthermore, this ratio can be altered by administering an agonist or a bifunctional antagonist.
Conclusion: Last 15 years have witnessed an explosive amount of work aimed at generating bifunctional compounds as a novel strategy to target GPCR homo- and heterodimers, including dopamine receptors. Their clinical use is far from trivial, but, at least, they have been used to validate the existence of receptor dimers in-vitro and in-vivo. The dualsteric compound SB269, 652, with its peculiar pharmacological profile, may offer therapeutic advantages and a better tolerability in comparison with pure antagonists at D2 and D3 receptors and pave the way for a new generation of antipsychotic drugs.
Keywords: G protein-coupled receptor (GPCR); and antipsychotics; bivalent ligand; dimerization; dopamine receptors; dualsteric ligand; single-molecule microscopy.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures


Similar articles
-
Distinctive binding properties of the negative allosteric modulator, [3H]SB269,652, at recombinant dopamine D3 receptors.Eur J Pharmacol. 2018 Jan 15;819:181-189. doi: 10.1016/j.ejphar.2017.12.002. Epub 2017 Dec 6. Eur J Pharmacol. 2018. PMID: 29223348
-
The First Negative Allosteric Modulator for Dopamine D2 and D3 Receptors, SB269652 May Lead to a New Generation of Antipsychotic Drugs.Mol Pharmacol. 2017 Jun;91(6):586-594. doi: 10.1124/mol.116.107607. Epub 2017 Mar 6. Mol Pharmacol. 2017. PMID: 28265019 Free PMC article. Review.
-
Bitropic D3 Dopamine Receptor Selective Compounds as Potential Antipsychotics.Curr Pharm Des. 2015;21(26):3700-24. doi: 10.2174/1381612821666150724100830. Curr Pharm Des. 2015. PMID: 26205291 Review.
-
Novel dimensions of D3 receptor function: Focus on heterodimerisation, transactivation and allosteric modulation.Eur Neuropsychopharmacol. 2015 Sep;25(9):1470-9. doi: 10.1016/j.euroneuro.2014.09.016. Epub 2014 Oct 23. Eur Neuropsychopharmacol. 2015. PMID: 25453482 Review.
-
Homobivalent Dopamine D2 Receptor Ligands Modulate the Dynamic Equilibrium of D2 Monomers and Homo- and Heterodimers.ACS Chem Biol. 2021 Feb 19;16(2):371-379. doi: 10.1021/acschembio.0c00895. Epub 2021 Jan 12. ACS Chem Biol. 2021. PMID: 33435665
Cited by
-
Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences.Pharmaceuticals (Basel). 2021 Mar 8;14(3):238. doi: 10.3390/ph14030238. Pharmaceuticals (Basel). 2021. PMID: 33800403 Free PMC article. Review.
-
The World of GPCR dimers - Mapping dopamine receptor D2 homodimers in different activation states and configuration arrangements.Comput Struct Biotechnol J. 2023 Sep 3;21:4336-4353. doi: 10.1016/j.csbj.2023.08.032. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37711187 Free PMC article.
-
Dopamine Receptor Subtypes, Physiology and Pharmacology: New Ligands and Concepts in Schizophrenia.Front Pharmacol. 2020 Jul 14;11:1003. doi: 10.3389/fphar.2020.01003. eCollection 2020. Front Pharmacol. 2020. PMID: 32765257 Free PMC article. Review.
-
Design of a True Bivalent Ligand with Picomolar Binding Affinity for a G Protein-Coupled Receptor Homodimer.J Med Chem. 2018 Oct 25;61(20):9335-9346. doi: 10.1021/acs.jmedchem.8b01249. Epub 2018 Oct 11. J Med Chem. 2018. PMID: 30257092 Free PMC article.
-
Dopamine D3 Receptor Heteromerization: Implications for Neuroplasticity and Neuroprotection.Biomolecules. 2020 Jul 9;10(7):1016. doi: 10.3390/biom10071016. Biomolecules. 2020. PMID: 32659920 Free PMC article. Review.
References
-
- Beaulieu J.M., Espinoza S., Gainetdinov R.R. Dopamine receptors - IUPHAR Review 13. Br. J. Pharmacol. 2015;172(1):1–23. [http://dx.doi.org/10.1111/bph.12906]. [PMID: 25671228]. - PMC - PubMed
-
- Missale C., Nash S.R., Robinson S.W., Jaber M., Caron M.G. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78(1):189–225. [PMID: 9457173]. - PubMed
-
- Blum K., Badgaiyan R.D., Agan G., Fratantonio J., Simpatico T., Febo M., Haberstick B.C., Smolen A., Gold M.S. Molecular genetic testing in reward deficiency syndrome (RDS): Facts and fiction. J Reward Defic Syndr. 2015;1(1):65–68. [http://dx.doi.org/ 10.17756/jrds.2015-009]. [PMID: 26052557]. - PMC - PubMed
-
- Maggio R., Novi F., Scarselli M., Corsini G.U. The impact of G-protein-coupled receptor hetero-oligomerization on function and pharmacology. FEBS J. 2005;272(12):2939–2946. [http://dx.doi. org/10.1111/j.1742-4658.2005.04729.x]. [PMID: 15955054]. - PubMed
-
- Maggio R., Scarselli M., Capannolo M., Millan M.J. Novel dimensions of D3 receptor function: Focus on heterodimerisation, transactivation and allosteric modulation. Eur. Neuropsychopharmacol. 2015;25(9):1470–1479. [http://dx.doi.org/10.1016/j. euroneuro.2014.09.016]. [PMID: 25453482]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
