Ultrasound and microbubble induced release from intracellular compartments
- PMID: 28521780
- PMCID: PMC5437622
- DOI: 10.1186/s12896-017-0364-3
Ultrasound and microbubble induced release from intracellular compartments
Abstract
Background: Ultrasound and microbubbles (USMB) have been shown to enhance the intracellular uptake of molecules, generally thought to occur as a result of sonoporation. The underlying mechanism associated with USMB-enhanced intracellular uptake such as membrane disruption and endocytosis may also be associated with USMB-induced release of cellular materials to the extracellular milieu. This study investigates USMB effects on the molecular release from cells through membrane-disruption and exocytosis.
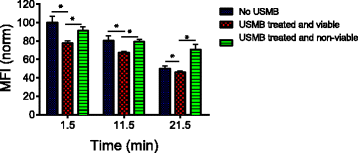
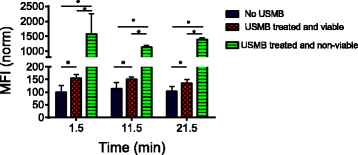
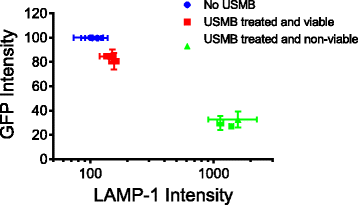
Results: USMB induced the release of 19% and 67% of GFP from the cytoplasm in viable and non-viable cells, respectively. Tfn release from early/recycling endosomes increased by 23% in viable cells upon USMB treatment. In addition, the MFI of LAMP-1 antibody increased by 50% in viable cells, suggesting USMB-stimulated lysosome exocytosis. In non-viable cells, labeling of LAMP-1 intracellular structures in the absence of cell permeabilization by detergents suggests that USMB-induced cell death correlates with lysosomal permeabilization.
Conclusions: In conclusion, USMB enhanced the molecular release from the cytoplasm, lysosomes, and early/recycling endosomes.
Keywords: Acoustic cavitation bioeffects; Cellular bioeffects of ultrasound; Endocytosis; Exocytosis; In vitro ultrasound bioeffects; Intracellular release; Intracellular uptake; Sonoporation; Ultrasound and microbubble.
Figures





Similar articles
-
Microbubbles-Assisted Ultrasound Triggers the Release of Extracellular Vesicles.Int J Mol Sci. 2017 Jul 25;18(8):1610. doi: 10.3390/ijms18081610. Int J Mol Sci. 2017. PMID: 28757579 Free PMC article.
-
Ultrasound Microbubble Treatment Enhances Clathrin-Mediated Endocytosis and Fluid-Phase Uptake through Distinct Mechanisms.PLoS One. 2016 Jun 8;11(6):e0156754. doi: 10.1371/journal.pone.0156754. eCollection 2016. PLoS One. 2016. PMID: 27275866 Free PMC article.
-
Efficient Gene Delivery by Sonoporation Is Associated with Microbubble Entry into Cells and the Clathrin-Dependent Endocytosis Pathway.Ultrasound Med Biol. 2015 Jul;41(7):1913-26. doi: 10.1016/j.ultrasmedbio.2015.03.010. Epub 2015 Apr 27. Ultrasound Med Biol. 2015. PMID: 25929996
-
Using ultrasound and microbubble to enhance the effects of conventional cancer therapies in clinical settings.Cancer Metastasis Rev. 2025 Mar 15;44(1):39. doi: 10.1007/s10555-025-10255-5. Cancer Metastasis Rev. 2025. PMID: 40088396 Free PMC article. Review.
-
Understanding ultrasound induced sonoporation: definitions and underlying mechanisms.Adv Drug Deliv Rev. 2014 Jun;72:49-64. doi: 10.1016/j.addr.2013.11.008. Epub 2013 Nov 21. Adv Drug Deliv Rev. 2014. PMID: 24270006 Review.
Cited by
-
Synergistic enhancement of cell death by triple combination therapy of docetaxel, ultrasound and microbubbles, and radiotherapy on PC3 a prostate cancer cell line.Heliyon. 2022 Aug 15;8(8):e10213. doi: 10.1016/j.heliyon.2022.e10213. eCollection 2022 Aug. Heliyon. 2022. PMID: 36033334 Free PMC article.
-
Treatment of glaucomatous optic nerve damage using ginsenoside Rg1 mediated by ultrasound targeted microbubble destruction.Exp Ther Med. 2018 Jan;15(1):300-304. doi: 10.3892/etm.2017.5386. Epub 2017 Oct 27. Exp Ther Med. 2018. PMID: 29375689 Free PMC article.
-
Ultrasound-Optimized Extraction and Multi-Target Mechanistic Analysis of Antioxidant and Hypoglycemic Effects of Amomum villosum Essential Oil.Foods. 2025 Aug 9;14(16):2772. doi: 10.3390/foods14162772. Foods. 2025. PMID: 40870685 Free PMC article.
-
Sonobiopsy for sensitive and spatially targeted molecular diagnosis through integrating ultrasound with liquid biopsy.NPJ Acoust. 2025;1(1):12. doi: 10.1038/s44384-025-00014-9. Epub 2025 Jul 3. NPJ Acoust. 2025. PMID: 40620711 Free PMC article. Review.
-
AMPK is required for recovery from metabolic stress induced by ultrasound microbubble treatment.iScience. 2022 Dec 28;26(2):105883. doi: 10.1016/j.isci.2022.105883. eCollection 2023 Feb 17. iScience. 2022. PMID: 36685038 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

