In vivo evaluation of microglia activation by intracranial iron overload in central pain after spinal cord injury
- PMID: 28521818
- PMCID: PMC5437601
- DOI: 10.1186/s13018-017-0578-z
In vivo evaluation of microglia activation by intracranial iron overload in central pain after spinal cord injury
Abstract
Background: Central pain (CP) is a common clinical problem in patients with spinal cord injury (SCI). Recent studies found the pathogenesis of CP was related to the remodeling of the brain. We investigate the roles of iron overload and subsequent microglia activate in the remodeling of the brain after SCI.
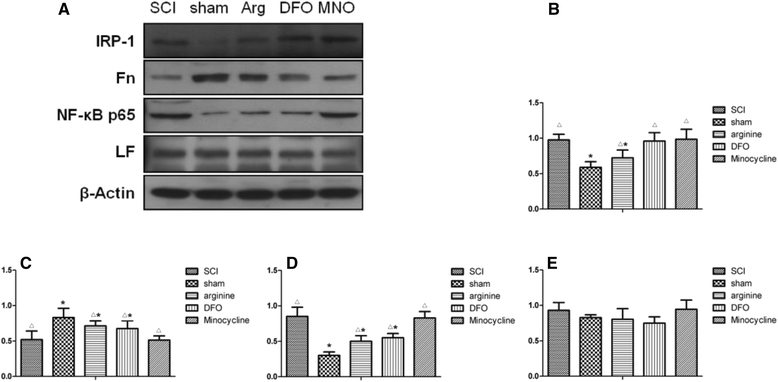
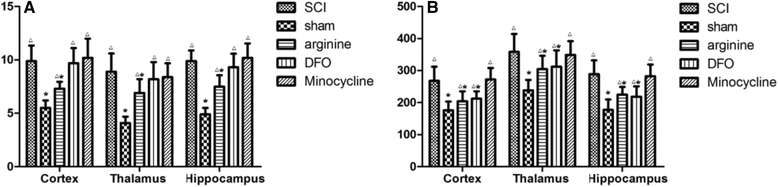
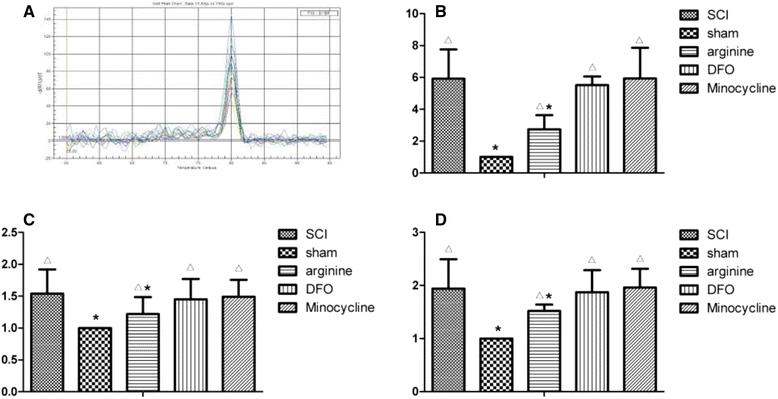
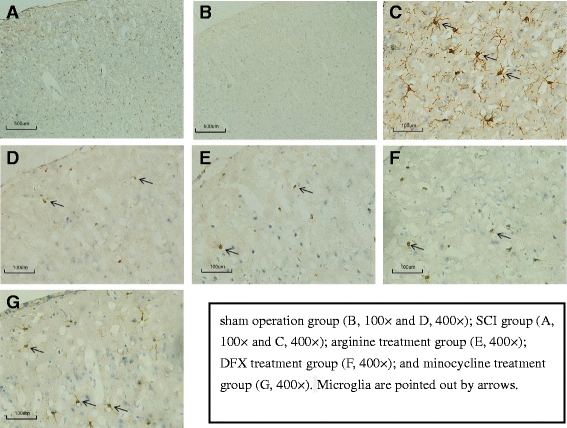
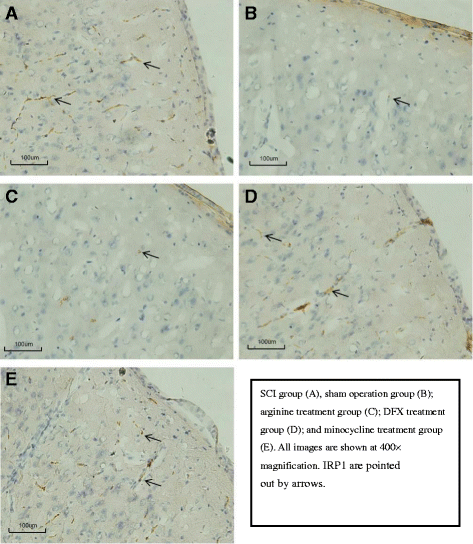
Methods: An SCI-induced CP model was established in Sprague-Dawley rats that were randomly assigned to SCI, sham operation, deferoxamine (DFX), minocycline, and nitric oxide synthase inhibitor treatment groups. At 12 weeks, pain behavior and thermal pain threshold were evaluated in each group, and iron transferrin receptor (TfR)1 and ferritin (Fn) mRNA, as well as iron-regulatory protein (IRP)1, FN, lactoferrin, and nuclear factor (NF)-κB protein levels in the rat brains were measured. Microglia proliferation and differentiation and IRP1 expression were evaluated by immunohistochemistry.
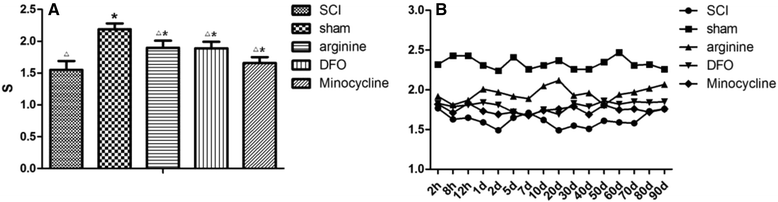
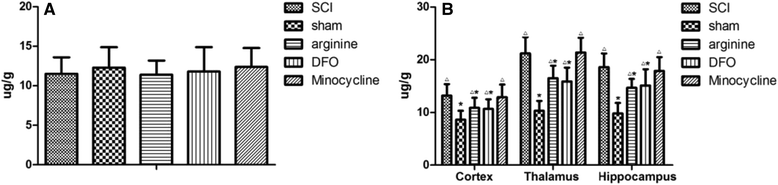
Results: Autophagy was observed in rats after SCI, accompanied by reduced latency of thermal pain, increased iron content and IRP1 and NF-κB levels in the hindlimb sensory area, hippocampus, and thalamus, and decreased Fn levels in the hindlimb sensory area. TfR1 mRNA expression was upregulated in activated microglia. Treatment with an iron-chelating agent, or inhibitors of nitric oxide synthase or microglia suppressed microglia proliferation.
Conclusions: SCI may induce intracranial iron overload, which activates microglia via NF-κB signaling. Microglia secrete inflammatory factors that induce neuronal damage and lead to CP. Treatment with an iron-chelating agent or NF-κB or microglia inhibitors can relieve CP resulting from SCI.
Keywords: Central pain; Iron; Microglia; NF-κB; Spinal cord injury.
Figures
Similar articles
-
Effect of oxidative stress induced by intracranial iron overload on central pain after spinal cord injury.J Orthop Surg Res. 2017 Feb 8;12(1):24. doi: 10.1186/s13018-017-0526-y. J Orthop Surg Res. 2017. PMID: 28178997 Free PMC article.
-
MiR-100 suppresses inflammatory activation of microglia and neuronal apoptosis following spinal cord injury via TLR4/NF-κB pathway.Eur Rev Med Pharmacol Sci. 2019 Oct;23(20):8713-8720. doi: 10.26355/eurrev_201910_19265. Eur Rev Med Pharmacol Sci. 2019. PMID: 31696457
-
Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury.J Neuroinflammation. 2020 Mar 20;17(1):90. doi: 10.1186/s12974-020-01751-2. J Neuroinflammation. 2020. PMID: 32192500 Free PMC article.
-
Downregulation of USP4 Promotes Activation of Microglia and Subsequent Neuronal Inflammation in Rat Spinal Cord After Injury.Neurochem Res. 2017 Nov;42(11):3245-3253. doi: 10.1007/s11064-017-2361-2. Epub 2017 Jul 28. Neurochem Res. 2017. PMID: 28755289
-
Neuroinflammatory contributions to pain after SCI: roles for central glial mechanisms and nociceptor-mediated host defense.Exp Neurol. 2014 Aug;258:48-61. doi: 10.1016/j.expneurol.2014.02.001. Exp Neurol. 2014. PMID: 25017887 Review.
Cited by
-
Main Cations and Cellular Biology of Traumatic Spinal Cord Injury.Cells. 2022 Aug 11;11(16):2503. doi: 10.3390/cells11162503. Cells. 2022. PMID: 36010579 Free PMC article. Review.
-
Exposure to quasi-ultrafine particulate matter accelerates memory impairment and Alzheimer's disease-like neuropathology in the AppNL-G-F knock-in mouse model.Toxicol Sci. 2023 May 31;193(2):175-191. doi: 10.1093/toxsci/kfad036. Toxicol Sci. 2023. PMID: 37074955 Free PMC article.
-
Receptor-Interacting Protein Kinase 3 Inhibition Relieves Mechanical Allodynia and Suppresses NLRP3 Inflammasome and NF-κB in a Rat Model of Spinal Cord Injury.Front Mol Neurosci. 2022 Apr 19;15:861312. doi: 10.3389/fnmol.2022.861312. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35514432 Free PMC article.
-
Insight into the potential role of ferroptosis in neurodegenerative diseases.Front Cell Neurosci. 2022 Oct 27;16:1005182. doi: 10.3389/fncel.2022.1005182. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36385946 Free PMC article. Review.
-
Model Senescent Microglia Induce Disease Related Changes in α-Synuclein Expression and Activity.Biomolecules. 2018 Aug 1;8(3):67. doi: 10.3390/biom8030067. Biomolecules. 2018. PMID: 30071596 Free PMC article.
References
-
- Bonica JJ. History of pain concepts and pain therapy. Mt Sinai J Med. 1991;58(3):191–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

