Linking hypoxia, DNA damage and proliferation in multicellular tumor spheroids
- PMID: 28521819
- PMCID: PMC5437385
- DOI: 10.1186/s12885-017-3319-0
Linking hypoxia, DNA damage and proliferation in multicellular tumor spheroids
Abstract
Background: Multicellular Tumor Spheroids are frequently used to mimic the regionalization of proliferation and the hypoxic environment within avascular tumors. Here we exploit these features to study the activation of DNA damage repair pathways and their correlation to developing hypoxia.
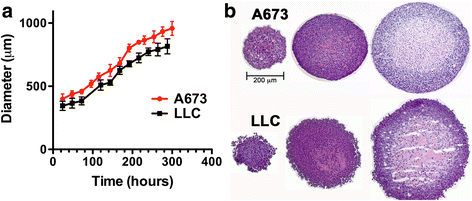
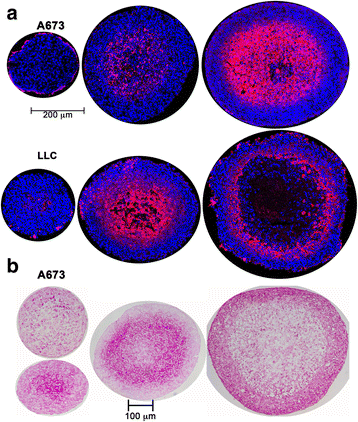
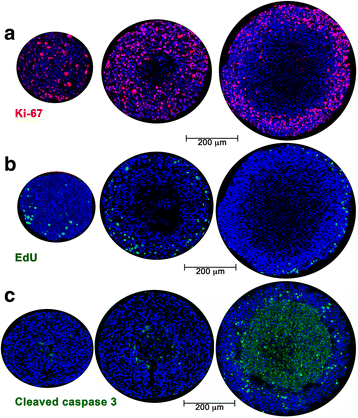
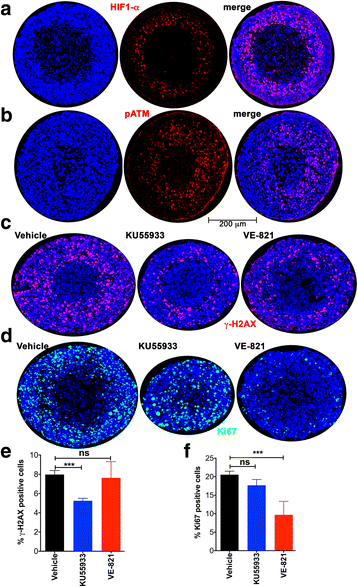
Methods: Activation of DNA damage repair markers, proliferation, cell death, glycogen accumulation and developing hypoxia were investigated using immunofluorescence, immuno-histochemistry, EdU incorporation, Western blots, COMET assays, and pharmacological agents in A673 Ewing sarcoma spheroids and monolayer cultures.
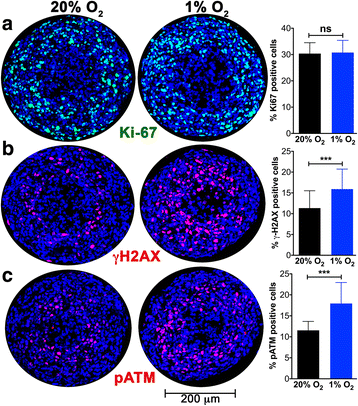
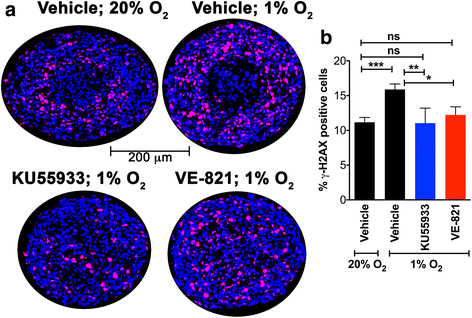
Results: DNA damage marker γ-H2AX is observed in the hypoxic, peri-necrotic region of growing spheroids. While most proliferating cells are seen on the spheroid surface, there are also a few Ki-67 positive cells in the hypoxic zone. The hypoxia-induced phosphorylation of H2AX to form γ-H2AX in spheroids is attenuated by the ATM inhibitor KU55933, but not the ATR inhibitor VE-821.
Conclusion: Tumor spheroids mimic tumor microenvironments such as the anoxic, hypoxic and oxic niches within solid tumors, as well as populations of cells that are viable, proliferating, and undergoing DNA damage repair processes under these different micro-environmental conditions. ATM, but not ATR, is the primary kinase responsible for γ-H2AX formation in the hypoxic core of A673 spheroids. Spheroids could offer unique advantages in testing therapeutics designed to target malignant cells that evade conventional treatment strategies by adapting to the hypoxic tumor microenvironment.
Keywords: ATM; ATR; DNA damage repair; Ewing sarcoma; Hypoxia; Proliferation; Spheroid.
Figures


References
-
- Brown JM. Tumor hypoxia in cancer therapy. Methods Enzymol. 2007;435:297–321. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

