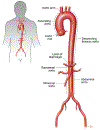
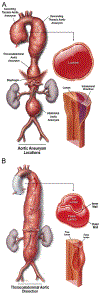
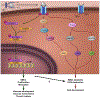
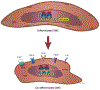
Molecular pathogenesis of genetic and sporadic aortic aneurysms and dissections
- PMID: 28521856
- PMCID: PMC7335366
- DOI: 10.1067/j.cpsurg.2017.01.001
Molecular pathogenesis of genetic and sporadic aortic aneurysms and dissections
Figures




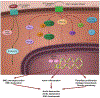
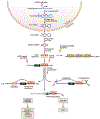
References
-
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd Improved prognosis of thoracic aortic aneurysms: a population-based study. J Am Med Assoc. 1998;280(22):1926–1929. - PubMed
-
- Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114(24):2611–2618. - PubMed
-
- Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111(6):816–828. - PubMed
-
- Meszaros I, Morocz J, Szlavi J, et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117(5):1271–1278. - PubMed
-
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79(2):176–180. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

