Harnessing the properties of dendritic cells in the pursuit of immunological tolerance
- PMID: 28521905
- PMCID: PMC6138597
- DOI: 10.1016/j.bj.2017.01.002
Harnessing the properties of dendritic cells in the pursuit of immunological tolerance
Abstract
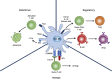
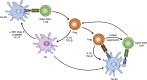
The acquisition of self-perpetuating, immunological tolerance specific for graft alloantigens has long been described as the "holy grail" of clinical transplantation. By removing the need for life-long immunosuppression following engraftment, the adverse consequences of immunosuppressive regimens, including chronic infections and malignancy, may be avoided. Furthermore, autoimmune diseases and allergy are, by definition, driven by aberrant immunological responses to ordinarily innocuous antigens. The re-establishment of permanent tolerance towards instigating antigens may, therefore, provide a cure to these common diseases. Whilst various cell types exhibiting a tolerogenic phenotype have been proposed for such a task, tolerogenic dendritic cells (tol-DCs) are exquisitely adapted for antigen presentation and interact with many facets of the immune system: as such, they are attractive candidates for use in strategies for immune intervention. We review here our current understanding of tol-DC mediated induction and maintenance of immunological tolerance. Additionally, we discuss recent in vitro findings from animal models and clinical trials of tol-DC immunotherapy in the setting of transplantation, autoimmunity and allergy which highlight their promising therapeutic potential, and speculate how tol-DC therapy may be developed in the future.
Keywords: Allograft rejection; Autoimmunity; Dendritic cell; Immunotherapy; Regulatory T cell; Tolerance.
Copyright © 2017 Chang Gung University. Published by Elsevier B.V. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

