Functional Properties of the Mitochondrial Carrier System
- PMID: 28522206
- PMCID: PMC5773108
- DOI: 10.1016/j.tcb.2017.04.004
Functional Properties of the Mitochondrial Carrier System
Abstract
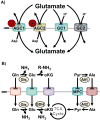
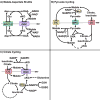
The mitochondrial carrier system (MCS) transports small molecules between mitochondria and the cytoplasm. It is integral to the core mitochondrial function to regulate cellular chemistry by metabolism. The mammalian MCS comprises the transporters of the 53-member canonical SLC25A family and a lesser number of identified noncanonical transporters. The recent discovery and investigations of the mitochondrial pyruvate carrier (MPC) illustrate the diverse effects a single mitochondrial carrier may exert on cellular function. However, the transport selectivities of many carriers remain unknown, and most have not been functionally investigated in mammalian cells. The mechanisms coordinating their function as a unified system remain undefined. Increased accessibility to molecular genetic and metabolomic technologies now greatly enables investigation of the MCS. Continued investigation of the MCS may reveal how mitochondria encode complex regulatory information within chemical thermodynamic gradients. This understanding may enable precision modulation of cellular chemistry to counteract the dysmetabolism inherent in disease.
Keywords: carrier; mitochondria; mitochondrial pyruvate carrier; redox cycles; systems biology; transporter.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Mitochondrial metabolite transport.Essays Biochem. 2010;47:37-52. doi: 10.1042/bse0470037. Essays Biochem. 2010. PMID: 20533899 Review.
-
Mitochondrial pyruvate carrier in Trypanosoma brucei.Mol Microbiol. 2016 May;100(3):442-56. doi: 10.1111/mmi.13325. Epub 2016 Feb 10. Mol Microbiol. 2016. PMID: 26748989
-
Mitochondrial pyruvate transport: a historical perspective and future research directions.Biochem J. 2015 Mar 15;466(3):443-54. doi: 10.1042/BJ20141171. Biochem J. 2015. PMID: 25748677 Free PMC article. Review.
-
Stress-seventy subfamily A 4, A member of HSP70, confers yeast cadmium tolerance in the loss of mitochondria pyruvate carrier 1.Plant Signal Behav. 2020;15(2):1719312. doi: 10.1080/15592324.2020.1719312. Epub 2020 Jan 27. Plant Signal Behav. 2020. PMID: 31985324 Free PMC article.
-
20,000 picometers under the OMM: diving into the vastness of mitochondrial metabolite transport.EMBO Rep. 2020 May 6;21(5):e50071. doi: 10.15252/embr.202050071. Epub 2020 Apr 23. EMBO Rep. 2020. PMID: 32329174 Free PMC article. Review.
Cited by
-
Drosophila melanogaster Mitochondrial Carriers: Similarities and Differences with the Human Carriers.Int J Mol Sci. 2020 Aug 22;21(17):6052. doi: 10.3390/ijms21176052. Int J Mol Sci. 2020. PMID: 32842667 Free PMC article. Review.
-
Mitochondrial pyruvate carrier 1: a novel prognostic biomarker that predicts favourable patient survival in cancer.Cancer Cell Int. 2021 May 31;21(1):288. doi: 10.1186/s12935-021-01996-8. Cancer Cell Int. 2021. PMID: 34059057 Free PMC article. Review.
-
Mitochondrial network responses in oxidative physiology and disease.Free Radic Biol Med. 2018 Feb 20;116:31-40. doi: 10.1016/j.freeradbiomed.2018.01.005. Epub 2018 Jan 6. Free Radic Biol Med. 2018. PMID: 29317273 Free PMC article. Review.
-
Mitochondrial pyruvate carriers are required for myocardial stress adaptation.Nat Metab. 2020 Nov;2(11):1248-1264. doi: 10.1038/s42255-020-00288-1. Epub 2020 Oct 26. Nat Metab. 2020. PMID: 33106689 Free PMC article.
-
An Asp to Strike Out Cancer? Therapeutic Possibilities Arising from Aspartate's Emerging Roles in Cell Proliferation and Survival.Biomolecules. 2021 Nov 10;11(11):1666. doi: 10.3390/biom11111666. Biomolecules. 2021. PMID: 34827664 Free PMC article. Review.
References
-
- Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med. 2013;34(2–3):465–84. - PubMed
-
- Palmieri F, Monne M. Discoveries, metabolic roles and diseases of mitochondrial carriers: A review. Biochim Biophys Acta. 2016;1863(10):2362–78. - PubMed
-
- Aquila H, et al. Complete amino acid sequence of the ADP/ATP carrier from beef heart mitochondria. Hoppe Seylers Z Physiol Chem. 1982;363(3):345–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

