The Drosophila LIN54 homolog Mip120 controls two aspects of oogenesis
- PMID: 28522430
- PMCID: PMC5550918
- DOI: 10.1242/bio.025825
The Drosophila LIN54 homolog Mip120 controls two aspects of oogenesis
Abstract
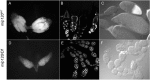
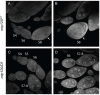
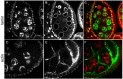
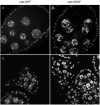
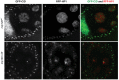
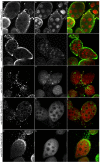
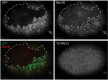
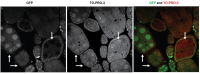
The conserved multi-protein MuvB core associates with the Myb oncoproteins and with the RB-E2F-DP tumor suppressor proteins in complexes that regulate cell proliferation, differentiation, and apoptosis. Drosophila Mip120, a homolog of LIN54, is a sequence-specific DNA-binding protein within the MuvB core. A mutant of Drosophilamip120 was previously shown to cause female and male sterility. We now show that Mip120 regulates two different aspects of oogenesis. First, in the absence of the Mip120 protein, egg chambers arrest during the transition from stage 7 to 8 with a failure of the normal program of chromosomal dynamics in the ovarian nurse cells. Specifically, the decondensation, disassembly and dispersion of the endoreplicated polytene chromosomes fail to occur without Mip120. The conserved carboxy-terminal DNA-binding and protein-protein interaction domains of Mip120 are necessary but not sufficient for this process. Second, we show that a lack of Mip120 causes a dramatic increase in the expression of benign gonial cell neoplasm (bgcn), a gene that is normally expressed in only a small number of cells within the ovary including the germline stem cells.
Keywords: Germline; LIN54; Mip120; Nurse cell; Oogenesis.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

