Structure of eIF4E in Complex with an eIF4G Peptide Supports a Universal Bipartite Binding Mode for Protein Translation
- PMID: 28522457
- PMCID: PMC5490897
- DOI: 10.1104/pp.17.00193
Structure of eIF4E in Complex with an eIF4G Peptide Supports a Universal Bipartite Binding Mode for Protein Translation
Abstract
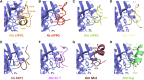
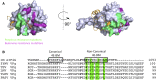
The association-dissociation of the cap-binding protein eukaryotic translation initiation factor 4E (eIF4E) with eIF4G is a key control step in eukaryotic translation. The paradigm on the eIF4E-eIF4G interaction states that eIF4G binds to the dorsal surface of eIF4E through a single canonical alpha-helical motif, while metazoan eIF4E-binding proteins (m4E-BPs) advantageously compete against eIF4G via bimodal interactions involving this canonical motif and a second noncanonical motif of the eIF4E surface. Metazoan eIF4Gs share this extended binding interface with m4E-BPs, with significant implications on the understanding of translation regulation and the design of therapeutic molecules. Here we show the high-resolution structure of melon (Cucumis melo) eIF4E in complex with a melon eIF4G peptide and propose the first eIF4E-eIF4G structural model for plants. Our structural data together with functional analyses demonstrate that plant eIF4G binds to eIF4E through both the canonical and noncanonical motifs, similarly to metazoan eIF4E-eIF4G complexes. As in the case of metazoan eIF4E-eIF4G, this may have very important practical implications, as plant eIF4E-eIF4G is also involved in a significant number of plant diseases. In light of our results, a universal eukaryotic bipartite mode of binding to eIF4E is proposed.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures








Similar articles
-
The Structures of eIF4E-eIF4G Complexes Reveal an Extended Interface to Regulate Translation Initiation.Mol Cell. 2016 Nov 3;64(3):467-479. doi: 10.1016/j.molcel.2016.09.020. Epub 2016 Oct 20. Mol Cell. 2016. PMID: 27773676
-
Structural motifs in eIF4G and 4E-BPs modulate their binding to eIF4E to regulate translation initiation in yeast.Nucleic Acids Res. 2018 Jul 27;46(13):6893-6908. doi: 10.1093/nar/gky542. Nucleic Acids Res. 2018. PMID: 30053226 Free PMC article.
-
4E-BPs require non-canonical 4E-binding motifs and a lateral surface of eIF4E to repress translation.Nat Commun. 2014 Sep 2;5:4790. doi: 10.1038/ncomms5790. Nat Commun. 2014. PMID: 25179781 Free PMC article.
-
Control of the eIF4E activity: structural insights and pharmacological implications.Cell Mol Life Sci. 2021 Nov;78(21-22):6869-6885. doi: 10.1007/s00018-021-03938-z. Epub 2021 Sep 19. Cell Mol Life Sci. 2021. PMID: 34541613 Free PMC article. Review.
-
Translational control of mRNAs by 3'-Untranslated region binding proteins.BMB Rep. 2017 Apr;50(4):194-200. doi: 10.5483/bmbrep.2017.50.4.040. BMB Rep. 2017. PMID: 28287067 Free PMC article. Review.
Cited by
-
What, where, and how: Regulation of translation and the translational landscape in plants.Plant Cell. 2024 May 1;36(5):1540-1564. doi: 10.1093/plcell/koad197. Plant Cell. 2024. PMID: 37437121 Free PMC article. Review.
-
Structural studies of the eIF4E-VPg complex reveal a direct competition for capped RNA: Implications for translation.Proc Natl Acad Sci U S A. 2019 Nov 26;116(48):24056-24065. doi: 10.1073/pnas.1904752116. Epub 2019 Nov 11. Proc Natl Acad Sci U S A. 2019. PMID: 31712417 Free PMC article.
-
A Novel Interaction Network Used by Potyviruses in Virus-Host Interactions at the Protein Level.Viruses. 2019 Dec 14;11(12):1158. doi: 10.3390/v11121158. Viruses. 2019. PMID: 31847316 Free PMC article.
-
Phosphorylation of Arabidopsis eIF4E and eIFiso4E by SnRK1 inhibits translation.FEBS J. 2019 Oct;286(19):3778-3796. doi: 10.1111/febs.14935. Epub 2019 Jun 3. FEBS J. 2019. PMID: 31120171 Free PMC article.
-
Properties of the ternary complex formed by yeast eIF4E, p20 and mRNA.Sci Rep. 2018 Apr 30;8(1):6707. doi: 10.1038/s41598-018-25273-3. Sci Rep. 2018. PMID: 29712996 Free PMC article.
References
-
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58: 1948–1954 - PubMed
-
- Aitken CE, Lorsch JR (2012) A mechanistic overview of translation initiation in eukaryotes. Nat Struct Mol Biol 19: 568–576 - PubMed
-
- Altmann M, Müller PP, Pelletier J, Sonenberg N, Trachsel H (1989) A mammalian translation initiation factor can substitute for its yeast homologue in vivo. J Biol Chem 264: 12145–12147 - PubMed
-
- Bah A, Vernon RM, Siddiqui Z, Krzeminski M, Muhandiram R, Zhao C, Sonenberg N, Kay LE, Forman-Kay JD (2015) Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch. Nature 519: 106–109 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

