Brain-derived neurotrophic factor and airway fibrosis in asthma
- PMID: 28522569
- PMCID: PMC5582935
- DOI: 10.1152/ajplung.00580.2016
Brain-derived neurotrophic factor and airway fibrosis in asthma
Abstract
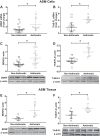
Airway remodeling in asthma driven by inflammation involves proliferation of epithelial cells and airway smooth muscle (ASM), as well as enhanced extracellular matrix (ECM) generation and deposition, i.e., fibrosis. Accordingly, understanding profibrotic mechanisms is important for developing novel therapeutic strategies in asthma. Recent studies, including our own, have suggested a role for locally produced growth factors such as brain-derived neurotrophic factor (BDNF) in mediating and modulating inflammation effects. In this study, we explored the profibrotic influence of BDNF in the context of asthma by examining expression, activity, and deposition of ECM proteins in primary ASM cells isolated from asthmatic vs. nonasthmatic patients. Basal BDNF expression and secretion, and levels of the high-affinity BDNF receptor TrkB, were higher in asthmatic ASM. Exogenous BDNF significantly increased ECM production and deposition, especially of collagen-1 and collagen-3 (less so fibronectin) and the activity of matrix metalloproteinases (MMP-2, MMP-9). Exposure to the proinflammatory cytokine TNFα significantly increased BDNF secretion, particularly in asthmatic ASM, whereas no significant changes were observed with IL-13. Chelation of BDNF using TrkB-Fc reversed TNFα-induced increase in ECM deposition. Conditioned media from asthmatic ASM enhanced ECM generation in nonasthmatic ASM, which was blunted by BDNF chelation. Inflammation-induced changes in MMP-2, MMP-9, and tissue inhibitor metalloproteinases (TIMP-1, TIMP-2) were reversed in the presence of TrkB-Fc. These novel data suggest ASM as an inflammation-sensitive source of BDNF within human airways, with autocrine effects on fibrosis relevant to asthma.
Keywords: airway smooth muscle; collagen; extracellular matrix; neurotrophin; tropomyosin-related kinase.
Copyright © 2017 the American Physiological Society.
Figures







References
-
- Braun A, Lommatzsch M, Mannsfeldt A, Neuhaus-Steinmetz U, Fischer A, Schnoy N, Lewin GR, Renz H. Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am J Respir Cell Mol Biol 21: 537–546, 1999. doi:10.1165/ajrcmb.21.4.3670. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

