Engineering macrophages to eat cancer: from "marker of self" CD47 and phagocytosis to differentiation
- PMID: 28522599
- PMCID: PMC6608056
- DOI: 10.1189/jlb.4RI1216-516R
Engineering macrophages to eat cancer: from "marker of self" CD47 and phagocytosis to differentiation
Abstract
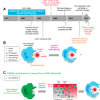
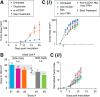
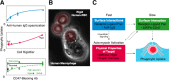
The ability of a macrophage to engulf and break down invading cells and other targets provides a first line of immune defense in nearly all tissues. This defining ability to "phagos" or devour can subsequently activate the entire immune system against foreign and diseased cells, and progress is now being made on a decades-old idea of directing macrophages to phagocytose specific targets, such as cancer cells. Engineered T cells provide precedence with recent clinical successes against liquid tumors, but solid tumors remain a challenge, and a handful of clinical trials seek to exploit the abundance of tumor-associated macrophages instead. Although macrophage differentiation into such phenotypes with deficiencies in phagocytic ability can raise challenges, newly recognized features of cancer cells that might be manipulated to increase the phagocytosis of those cells include ≥1 membrane protein, CD47, which broadly inhibits phagocytosis and is abundantly expressed on all healthy cells. Physical properties of the target also influence phagocytosis and again relate-via cytoskeleton forces-to differentiation pathways in solid tumors. Such pathways extend to mechanosensing by the nuclear lamina, which is known to influence signaling by soluble retinoids that can regulate the macrophage SIRPα, the receptor for CD47. Here, we highlight some of those past, present, and rapidly emerging efforts to understand and control macrophages for cancer therapy.
Keywords: cytoskeleton; mechanobiology; solid tumors.
© Society for Leukocyte Biology.
Figures

References
-
- Gosselin, D. , Link, V. M. , Romanoski, C. E. , Fonseca, G. J. , Eichenfield, D. Z. , Spann, N. J. , Stender, J. D. , Chun, H. B. , Garner, H. , Geissmann, F. , Glass, C. K. (2014) Environment drives selection and function of enhancers controlling tissue‐specific macrophage identities. Cell 159, 1327–1340. - PMC - PubMed
-
- Fidler, I. J. , Kleinerman, E. S. (1984) Lymphokine‐activated human blood monocytes destroy tumor cells but not normal cells under cocultivation conditions. J. Clin. Oncol. 2, 937–943. - PubMed
-
- Condeelis, J. , Pollard, J. W. (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124, 263–266. - PubMed
-
- Lu‐Emerson, C. , Snuderl, M. , Kirkpatrick, N. D. , Goveia, J. , Davidson, C. , Huang, Y. , Riedemann, L. , Taylor, J. , Ivy, P. , Duda, D. G. , Ancukiewicz, M. , Plotkin, S. R. , Chi, A. S. , Gerstner, E. R. , Eichler, A. F. , Dietrich, J. , Stemmer‐Rachamimov, A. O. , Batchelor, T. T. , Jain, R. K. (2013) Increase in tumor‐associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro Oncol. 15, 1079–1087. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

