Protective Effects of Hydrogen-Rich Saline Against Lipopolysaccharide-Induced Alveolar Epithelial-to-Mesenchymal Transition and Pulmonary Fibrosis
- PMID: 28522797
- PMCID: PMC5445901
- DOI: 10.12659/msm.900452
Protective Effects of Hydrogen-Rich Saline Against Lipopolysaccharide-Induced Alveolar Epithelial-to-Mesenchymal Transition and Pulmonary Fibrosis
Abstract
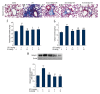
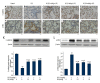
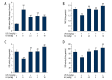
BACKGROUND Fibrotic change is one of the important reasons for the poor prognosis of patients with acute respiratory distress syndrome (ARDS). The present study investigated the effects of hydrogen-rich saline, a selective hydroxyl radical scavenger, on lipopolysaccharide (LPS)-induced pulmonary fibrosis. MATERIAL AND METHODS Male ICR mice were divided randomly into 5 groups: Control, LPS-treated plus vehicle treatment, and LPS-treated plus hydrogen-rich saline (2.5, 5, or 10 ml/kg) treatment. Twenty-eight days later, fibrosis was assessed by determination of collagen deposition, hydroxyproline, and type I collagen levels. Development of epithelial-to-mesenchymal transition (EMT) was identified by examining protein expressions of E-cadherin and α-smooth muscle actin (α-SMA). Transforming growth factor (TGF)-β1 content, total antioxidant capacity (T-AOC), malondialdehyde (MDA) content, catalase (CAT), and superoxide dismutase (SOD) activity were determined. RESULTS Mice exhibited increases in collagen deposition, hydroxyproline, type I collagen contents, and TGF-β1 production in lung tissues after LPS treatment. LPS-induced lung fibrosis was associated with increased expression of α-SMA, as well as decreased expression of E-cadherin. In addition, LPS treatment increased MDA levels but decreased T-AOC, CAT, and SOD activities in lung tissues, indicating that LPS induced pulmonary oxidative stress. Hydrogen-rich saline treatment at doses of 2.5, 5, or 10 ml/kg significantly attenuated LPS-induced pulmonary fibrosis. LPS-induced loss of E-cadherin in lung tissues was largely reversed, whereas the acquisition of α-SMA was dramatically decreased by hydrogen-rich saline treatment. In addition, hydrogen-rich saline treatment significantly attenuated LPS-induced oxidative stress. CONCLUSIONS Hydrogen-rich saline may protect against LPS-induced EMT and pulmonary fibrosis through suppressing oxidative stress.
Conflict of interest statement
The authors have not disclosed any potential conflicts of interest.
Figures

Similar articles
-
Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-β1 signaling.Clin Nutr. 2015 Aug;34(4):752-60. doi: 10.1016/j.clnu.2014.08.014. Epub 2014 Sep 6. Clin Nutr. 2015. PMID: 25234611
-
Hydrogen inhalation attenuated bleomycin-induced pulmonary fibrosis by inhibiting transforming growth factor-β1 and relevant oxidative stress and epithelial-to-mesenchymal transition.Exp Physiol. 2019 Dec;104(12):1942-1951. doi: 10.1113/EP088028. Epub 2019 Oct 23. Exp Physiol. 2019. PMID: 31535412
-
Edaravone attenuates lipopolysaccharide-induced acute respiratory distress syndrome associated early pulmonary fibrosis via amelioration of oxidative stress and transforming growth factor-β1/Smad3 signaling.Biochem Biophys Res Commun. 2018 Jan 1;495(1):706-712. doi: 10.1016/j.bbrc.2017.10.165. Epub 2017 Nov 2. Biochem Biophys Res Commun. 2018. PMID: 29102631
-
Mesenchymal Stromal Cells Derived Conditioned Medium in Pulmonary Fibrosis: A Systematic Review and Meta-analysis.Arch Iran Med. 2020 Dec 1;23(12):870-879. doi: 10.34172/aim.2020.116. Arch Iran Med. 2020. PMID: 33356346
-
The role of natural products in the prevention and treatment of pulmonary fibrosis: a review.Food Funct. 2021 Feb 15;12(3):990-1007. doi: 10.1039/d0fo03001e. Food Funct. 2021. PMID: 33459740 Review.
Cited by
-
N-acetylcysteine tiherapeutically protects against pulmonary fibrosis in a mouse model of silicosis.Biosci Rep. 2019 Jul 18;39(7):BSR20190681. doi: 10.1042/BSR20190681. Print 2019 Jul 31. Biosci Rep. 2019. PMID: 31273057 Free PMC article.
-
Molecular Hydrogen in the Treatment of Respiratory Diseases.Int J Mol Sci. 2025 Apr 26;26(9):4116. doi: 10.3390/ijms26094116. Int J Mol Sci. 2025. PMID: 40362357 Free PMC article. Review.
-
Evaluating the Therapeutic Mechanisms of Selected Active Compounds in Houttuynia cordata Thunb. in Pulmonary Fibrosis via Network Pharmacology Analysis.Front Pharmacol. 2021 Sep 30;12:733618. doi: 10.3389/fphar.2021.733618. eCollection 2021. Front Pharmacol. 2021. PMID: 34658873 Free PMC article.
-
Recent Advances in Studies of Molecular Hydrogen against Sepsis.Int J Biol Sci. 2019 May 11;15(6):1261-1275. doi: 10.7150/ijbs.30741. eCollection 2019. Int J Biol Sci. 2019. PMID: 31223285 Free PMC article. Review.
-
H2 Protects Against Lipopolysaccharide-Induced Cardiac Dysfunction via Blocking TLR4-Mediated Cytokines Expression.Front Pharmacol. 2019 Aug 5;10:865. doi: 10.3389/fphar.2019.00865. eCollection 2019. Front Pharmacol. 2019. PMID: 31440160 Free PMC article.
References
-
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. - PubMed
-
- Tomashefski JF., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–66. - PubMed
-
- Rocco PR, Dos Santos C, Pelosi P. Lung parenchyma remodeling in acute respiratory distress syndrome. Minerva Anestesiol. 2009;75:730–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

