Near-Infrared Heptamethine Cyanine Based Iron Oxide Nanoparticles for Tumor Targeted Multimodal Imaging and Photothermal Therapy
- PMID: 28522841
- PMCID: PMC5437012
- DOI: 10.1038/s41598-017-01108-5
Near-Infrared Heptamethine Cyanine Based Iron Oxide Nanoparticles for Tumor Targeted Multimodal Imaging and Photothermal Therapy
Abstract
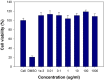
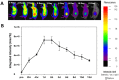
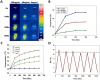
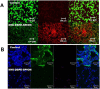
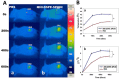
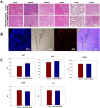
Near-infrared fluorescent (NIRF) imaging modality holds great promise for tumor detection and offers several advantages of bioimaging, such as high tissue penetration with less background scattering. The disadvantage of NIRF bioimaging is that it has very low spatial resolution. Thus, the combination of NIRF with magnetic resonance imaging (MRI) is a good option because MRI can provide anatomical information with a higher resolution. Heptamethine cyanine dye (MHI-148) has been reported to have tumor-targeting capability which was used here as the NIRF agent. DSPE-SPION nanoparticles were synthesized by the solvent hydration method and conjugated with MHI-148 dye to form a MRI/NIRF dual imaging probe. The size and charge of the MHI-DSPE-SPION were found to be about 84 ± 6 nm and 3.7 mV by DLS & Zeta Potential analysis. In vivo MRI of the SCC7 tumor showed an enhanced accumulation of MHI-DSPE-SPION, peaking at day 1, compared to 4 hrs with the control DSPE-SPION. An in vivo photothermal tumor reduction study was done on the SCC7 tumor of BALB/c nude mice. Tumor reduction study showed complete tumor removal after 8 days. In conclusion, MHI-DSPE-SPION can be used as a cancer theranostics material because it provides MRI-optical imaging capabilities and the photothermal therapy (PTT) effect.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

