The F-Actin Binding Protein Cortactin Regulates the Dynamics of the Exocytotic Fusion Pore through its SH3 Domain
- PMID: 28522963
- PMCID: PMC5415606
- DOI: 10.3389/fncel.2017.00130
The F-Actin Binding Protein Cortactin Regulates the Dynamics of the Exocytotic Fusion Pore through its SH3 Domain
Abstract
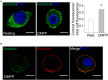
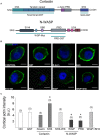
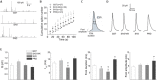
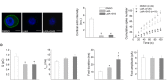
Upon cell stimulation, the network of cortical actin filaments is rearranged to facilitate the neurosecretory process. This actin rearrangement includes both disruption of the preexisting actin network and de novo actin polymerization. However, the mechanism by which a Ca2+ signal elicits the formation of new actin filaments remains uncertain. Cortactin, an actin-binding protein that promotes actin polymerization in synergy with the nucleation promoting factor N-WASP, could play a key role in this mechanism. We addressed this hypothesis by analyzing de novo actin polymerization and exocytosis in bovine adrenal chromaffin cells expressing different cortactin or N-WASP domains, or cortactin mutants that fail to interact with proline-rich domain (PRD)-containing proteins, including N-WASP, or to be phosphorylated by Ca2+-dependent kinases, such as ERK1/2 and Src. Our results show that the activation of nicotinic receptors in chromaffin cells promotes cortactin translocation to the cell cortex, where it colocalizes with actin filaments. We further found that, in association with PRD-containing proteins, cortactin contributes to the Ca2+-dependent formation of F-actin, and regulates fusion pore dynamics and the number of exocytotic events induced by activation of nicotinic receptors. However, whereas the actions of cortactin on the fusion pore dynamics seems to depend on the availability of monomeric actin and its phosphorylation by ERK1/2 and Src kinases, cortactin regulates the extent of exocytosis by a mechanism independent of actin polymerization. Together our findings point out a role for cortactin as a critical modulator of actin filament formation and exocytosis in neuroendocrine cells.
Keywords: N-WASP; actin polymerization; catecholamines; chromaffin cells; cortactin; exocytosis; fusion pore; neuroendocrine cells.
Figures


References
-
- Alés E., Tabares L., Poyato J. M., Valero V., Lindau M., Alvarez de Toledo G. (1999). High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat. Cell Biol. 1 40–44. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

