Dysbiosis of Intestinal Microbiota and Decreased Antimicrobial Peptide Level in Paneth Cells during Hypertriglyceridemia-Related Acute Necrotizing Pancreatitis in Rats
- PMID: 28522995
- PMCID: PMC5415626
- DOI: 10.3389/fmicb.2017.00776
Dysbiosis of Intestinal Microbiota and Decreased Antimicrobial Peptide Level in Paneth Cells during Hypertriglyceridemia-Related Acute Necrotizing Pancreatitis in Rats
Abstract
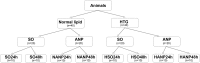
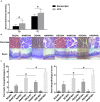
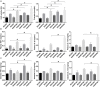
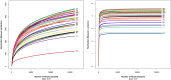
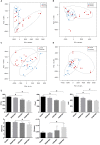
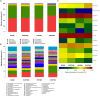
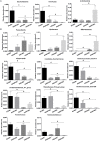
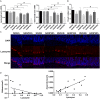
Hypertriglyceridemia (HTG) aggravates the course of acute pancreatitis (AP). Intestinal barrier dysfunction is implicated in the pathogenesis of AP during which dysbiosis of intestinal microbiota contributes to the dysfunction in intestinal barrier. However, few studies focus on the changes in intestine during HTG-related acute necrotizing pancreatitis (ANP). Here, we investigated the changes in intestinal microbiota and Paneth cell antimicrobial peptides (AMPs) in HTG-related ANP (HANP) in rats. Rats fed a high-fat diet to induce HTG and ANP was induced by retrograde injection of 3.5% sodium taurocholate into biliopancreatic duct. Rats were sacrificed at 24 and 48 h, respectively. Pancreatic and ileal injuries were evaluated by histological scores. Intestinal barrier function was assessed by plasma diamine oxidase activity and D-lactate level. Systemic and intestinal inflammation was evaluated by tumor necrosis factor alpha (TNFα), interleukin (IL)-1β, and IL-17A expression. 16S rRNA high throughput sequencing was used to investigate changes in intestinal microbiota diversity and structure. AMPs (α-defensin5 and lysozyme) expression was measured by real-time polymerase chain reaction (PCR) and immunofluorescence. The results showed that compared with those of normal-lipid ANP (NANP) groups, the HANP groups had more severe histopathological injuries in pancreas and distal ileum, aggravated intestinal barrier dysfunction and increased TNFα, IL-1β, and IL-17A expression in plasma and distal ileum. Principal component analysis showed structural segregation between the HANP and NANP group. α-Diversity estimators in the HANP group revealed decreased microbiota diversity compared with that in NANP group. Taxonomic analysis showed dysbiosis of intestinal microbiota structure. In the HANP group, at phyla level, Candidatus_Saccharibacteria and Tenericutes decreased significantly, whereas Actinobacteria increased. At genus level, Allobaculum, Bifidobacterium, and Parasutterella increased significantly, while Alloprevotella, Anaerotruncus, Candidatus_Saccharimonas, Christensenellaceae_R-7_group, Rikenellaceae_RC9_gut_group, Ruminiclostridium_5, Ruminococcaceae_UCG-005, and Ruminococcaceae_UCG-014 decreased. Compared with those in the NANP rats, mRNA expression of lysozyme and α-defensin5 and protein expression of lysozyme decreased significantly in the HANP rats. Moreover, in the NANP rats and the HANP rats, Allobaculum abundance was inversely correlated with lysozyme expression, while Anaerotruncus abundance was positively correlated with it by Spearman test. In conclusion, intestinal microbiota dysbiosis and decreased AMPs of Paneth cells might participate in the pathogenesis of intestinal barrier dysfunction in HANP.
Keywords: Paneth cell; acute necrotizing pancreatitis; antimicrobial peptides; hypertriglyceridemia; intestinal microbiota.
Figures
Similar articles
-
Gut microbiota interacts with inflammatory responses in acute pancreatitis.Therap Adv Gastroenterol. 2023 Oct 10;16:17562848231202133. doi: 10.1177/17562848231202133. eCollection 2023. Therap Adv Gastroenterol. 2023. PMID: 37829561 Free PMC article. Review.
-
Dysbiosis of intestinal microbiota and decrease in paneth cell antimicrobial peptide level during acute necrotizing pancreatitis in rats.PLoS One. 2017 Apr 25;12(4):e0176583. doi: 10.1371/journal.pone.0176583. eCollection 2017. PLoS One. 2017. PMID: 28441432 Free PMC article.
-
Paneth Cell Ablation Aggravates Pancreatic and Intestinal Injuries in a Rat Model of Acute Necrotizing Pancreatitis after Normal and High-Fat Diet.Mediators Inflamm. 2019 Nov 11;2019:8474523. doi: 10.1155/2019/8474523. eCollection 2019. Mediators Inflamm. 2019. PMID: 31827383 Free PMC article.
-
Commensal Escherichia coli Aggravates Acute Necrotizing Pancreatitis through Targeting of Intestinal Epithelial Cells.Appl Environ Microbiol. 2019 May 30;85(12):e00059-19. doi: 10.1128/AEM.00059-19. Print 2019 Jun 15. Appl Environ Microbiol. 2019. PMID: 30979838 Free PMC article.
-
Dysbiosis--a consequence of Paneth cell dysfunction.Semin Immunol. 2013 Nov 30;25(5):334-41. doi: 10.1016/j.smim.2013.09.006. Epub 2013 Nov 14. Semin Immunol. 2013. PMID: 24239045 Review.
Cited by
-
Bacteroides ovatus-mediated CD27- MAIT cell activation is associated with obesity-related T2D progression.Cell Mol Immunol. 2022 Jul;19(7):791-804. doi: 10.1038/s41423-022-00871-4. Epub 2022 May 11. Cell Mol Immunol. 2022. PMID: 35545662 Free PMC article.
-
Gut microbiota interacts with inflammatory responses in acute pancreatitis.Therap Adv Gastroenterol. 2023 Oct 10;16:17562848231202133. doi: 10.1177/17562848231202133. eCollection 2023. Therap Adv Gastroenterol. 2023. PMID: 37829561 Free PMC article. Review.
-
Updated review of research on the role of the gut microbiota and microbiota-derived metabolites in acute pancreatitis progression and inflammation-targeted therapy.Int J Biol Sci. 2025 Jan 20;21(3):1242-1258. doi: 10.7150/ijbs.108858. eCollection 2025. Int J Biol Sci. 2025. PMID: 39897025 Free PMC article. Review.
-
Impaired Abcb1a function and red meat in a translational colitis mouse model induces inflammation and alters microbiota composition.Front Med (Lausanne). 2023 Jul 31;10:1200317. doi: 10.3389/fmed.2023.1200317. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37588005 Free PMC article.
-
Study of Jianpi Mixture on Intestinal Microbiota of Diarrhea Irritable Bowel Syndrome Mice.Evid Based Complement Alternat Med. 2020 Apr 30;2020:5241308. doi: 10.1155/2020/5241308. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32419810 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

