Quantum Chemical Modeling of Hydrogen Bonding in Ionic Liquids
- PMID: 28523638
- PMCID: PMC5480408
- DOI: 10.1007/s41061-017-0142-7
Quantum Chemical Modeling of Hydrogen Bonding in Ionic Liquids
Abstract
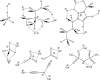
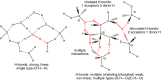
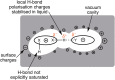
Hydrogen bonding (H-bonding) is an important and very general phenomenon. H-bonding is part of the basis of life in DNA, key in controlling the properties of water and ice, and critical to modern applications such as crystal engineering, catalysis applications, pharmaceutical and agrochemical development. H-bonding also plays a significant role for many ionic liquids (IL), determining the secondary structuring and affecting key physical parameters. ILs exhibit a particularly diverse and wide range of traditional as well as non-standard forms of H-bonding, in particular the doubly ionic H-bond is important. Understanding the fundamental nature of the H-bonds that form within ILs is critical, and one way of accessing this information, that cannot be recovered by any other computational method, is through quantum chemical electronic structure calculations. However, an appropriate method and basis set must be employed, and a robust procedure for determining key structures is essential. Modern generalised solvation models have recently been extended to ILs, bringing both advantages and disadvantages. QC can provide a range of information on geometry, IR and Raman spectra, NMR spectra and at a more fundamental level through analysis of the electronic structure.
Keywords: DFT; Hydrogen bonding; Ionic liquids.
Figures
Similar articles
-
A step towards the a priori design of ionic liquids.Phys Chem Chem Phys. 2013 Jul 21;15(27):11566-78. doi: 10.1039/c3cp50521a. Epub 2013 Jun 10. Phys Chem Chem Phys. 2013. PMID: 23752557
-
Hydrogen-bonding and the dissolution mechanism of uracil in an acetate ionic liquid: new insights from NMR spectroscopy and quantum chemical calculations.J Phys Chem B. 2013 Apr 18;117(15):4109-20. doi: 10.1021/jp400749j. Epub 2013 Apr 4. J Phys Chem B. 2013. PMID: 23521702
-
Hydrogen bonding in ionic liquids.Chem Soc Rev. 2015 Mar 7;44(5):1257-88. doi: 10.1039/c4cs00278d. Chem Soc Rev. 2015. PMID: 25582457
-
Understanding ionic liquids through atomistic and coarse-grained molecular dynamics simulations.Acc Chem Res. 2007 Nov;40(11):1193-9. doi: 10.1021/ar700160p. Epub 2007 Oct 13. Acc Chem Res. 2007. PMID: 17935302 Review.
-
Hydrogen-Bonding Motifs in Hydroxy-Functionalized Ionic Liquids.Annu Rev Phys Chem. 2025 Apr;76(1):589-614. doi: 10.1146/annurev-physchem-082423-020307. Annu Rev Phys Chem. 2025. PMID: 40258243 Review.
Cited by
-
Influence of Anion and Cation Structure of Ionic Liquids on Carboxylic Acids Extraction.Front Chem. 2019 Mar 14;7:117. doi: 10.3389/fchem.2019.00117. eCollection 2019. Front Chem. 2019. PMID: 30923706 Free PMC article.
-
Infrared Spectroscopy in the Middle Frequency Range for Various Imidazolium Ionic Liquids-Common Spectroscopic Characteristics of Vibrational Modes with In-Plane +C(2)-H and +C(4,5)-H Bending Motions and Peak Splitting Behavior Due to Local Symmetry Breaking of Vibrational Modes of the Tetrafluoroborate Anion.ACS Omega. 2021 Jan 8;6(2):1709-1717. doi: 10.1021/acsomega.0c05769. eCollection 2021 Jan 19. ACS Omega. 2021. PMID: 33490829 Free PMC article.
-
Infrared and Terahertz Spectroscopic Investigation of Imidazolium, Pyridinium, and Tetraalkylammonium Tetrafluoroborate Ionic Liquids.ACS Omega. 2022 Aug 22;7(34):29804-29812. doi: 10.1021/acsomega.2c02601. eCollection 2022 Aug 30. ACS Omega. 2022. PMID: 36061654 Free PMC article.
-
Structural Features of Triethylammonium Acetate through Molecular Dynamics.Molecules. 2020 Mar 21;25(6):1432. doi: 10.3390/molecules25061432. Molecules. 2020. PMID: 32245229 Free PMC article.
-
Influence of Ionic Liquid Film Thickness and Flow Rate on Macrocyclization Efficiency and Selectivity in Supported Ionic Liquid-Liquid Phase Catalysis.Chemistry. 2025 Jan 14;31(3):e202403237. doi: 10.1002/chem.202403237. Epub 2024 Dec 11. Chemistry. 2025. PMID: 39585183 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources