Closed-loop neuromodulation systems: next-generation treatments for psychiatric illness
- PMID: 28523978
- PMCID: PMC5461950
- DOI: 10.1080/09540261.2017.1282438
Closed-loop neuromodulation systems: next-generation treatments for psychiatric illness
Abstract
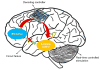
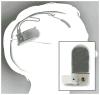
Despite deep brain stimulation's positive early results in psychiatric disorders, well-designed clinical trials have yielded inconsistent clinical outcomes. One path to more reliable benefit is closed-loop therapy: stimulation that is automatically adjusted by a device or algorithm in response to changes in the patient's electrical brain activity. These interventions may provide more precise and patient-specific treatments. This article first introduces the available closed-loop neuromodulation platforms, which have shown clinical efficacy in epilepsy and strong early results in movement disorders. It discusses the strengths and limitations of these devices in the context of psychiatric illness. It then describes emerging technologies to address these limitations, including pre-clinical developments such as wireless deep neurostimulation and genetically targeted neuromodulation. Finally, ongoing challenges and limitations for closed-loop psychiatric brain stimulation development, most notably the difficulty of identifying meaningful biomarkers for titration, are discussed. This is considered in the recently-released Research Domain Criteria (RDoC) framework, and how neuromodulation and RDoC are jointly very well suited to address the problem of treatment-resistant illness is described.
Keywords: Deep brain stimulation; bioengineering; biomarker; electrophysiology; neuromodulation; transdiagnostic.
Conflict of interest statement
Preparation of this work was supported in part by grants from the Brain & Behavior Research Foundation, Harvard Brain Initiative, Defense Advanced Research Projects Agency (cooperative agreement W911NF-14-2-0045, issued by the Army Research Office contracting office in support of DARPA’s SUBNETS program), and National Institute of Mental Health (MH109722-01). The views, opinions, and/or findings expressed are those of the author(s) and should not be interpreted as representing the official views or policies of the Department of Defense, the U.S. Government. ASW has consulted for and receives device donations from Medtronic, which manufactures devices discussed in the article. ASW and ML are named inventors on patent applications related to closed-loop neurostimulation.
Figures
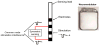
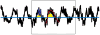
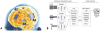
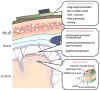
References
-
- Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nature Methods. 2013;10(5):413–420. http://doi.org/10.1038/nmeth.2434. - DOI - PubMed
-
- Aston-Jones G, Deisseroth K. Recent advances in optogenetics and pharmacogenetics. Brain Research. 2013 http://doi.org/10.1016/j.brainres.2013.01.026. - DOI - PMC - PubMed
-
- Bentley JN, Chestek C, Stacey WC, Patil PG. Optogenetics in epilepsy. Neurosurgical Focus. 2013;34(June):E4. http://doi.org/10.3171/2013.3.FOCUS1364. - DOI - PubMed
-
- Bergfeld IO, Mantione M, Hoogendoorn MLC, Ruhé HG, Notten P, Van Laarhoven J, … Denys D. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2016;73(5):456–64. http://doi.org/10.1001/jamapsychiatry.2016.0152. - DOI - PubMed
-
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, … Schlaepfer TE. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biological Psychiatry. 2010;67(2):110–6. http://doi.org/10.1016/j.biopsych.2009.09.013. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
