The Mycelium as a Network
- PMID: 28524023
- PMCID: PMC11687498
- DOI: 10.1128/microbiolspec.FUNK-0033-2017
The Mycelium as a Network
Abstract
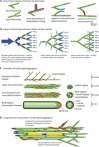
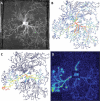
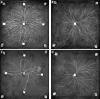
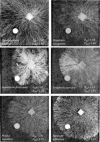
The characteristic growth pattern of fungal mycelia as an interconnected network has a major impact on how cellular events operating on a micron scale affect colony behavior at an ecological scale. Network structure is intimately linked to flows of resources across the network that in turn modify the network architecture itself. This complex interplay shapes the incredibly plastic behavior of fungi and allows them to cope with patchy, ephemeral resources, competition, damage, and predation in a manner completely different from multicellular plants or animals. Here, we try to link network structure with impact on resource movement at different scales of organization to understand the benefits and challenges of organisms that grow as connected networks. This inevitably involves an interdisciplinary approach whereby mathematical modeling helps to provide a bridge between information gleaned by traditional cell and molecular techniques or biophysical approaches at a hyphal level, with observations of colony dynamics and behavior at an ecological level.
Figures




References
-
- Buller AHR. 1924. Researches on Fungi, vol 3. Longmans Green, London, United Kingdom.
-
- Buller AHR. 1931. Researches on Fungi, vol 4. Longmans Green, London, United Kingdom.
-
- Buller AHR. 1933. Researches on Fungi, vol 5. Longmans Green, London, United Kingdom.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

