Modulators of microglial activation and polarization after intracerebral haemorrhage
- PMID: 28524175
- PMCID: PMC5575938
- DOI: 10.1038/nrneurol.2017.69
Modulators of microglial activation and polarization after intracerebral haemorrhage
Abstract
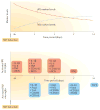
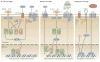
Intracerebral haemorrhage (ICH) is the most lethal subtype of stroke but currently lacks effective treatment. Microglia are among the first non-neuronal cells on the scene during the innate immune response to ICH. Microglia respond to acute brain injury by becoming activated and developing classic M1-like (proinflammatory) or alternative M2-like (anti-inflammatory) phenotypes. This polarization implies as yet unrecognized actions of microglia in ICH pathology and recovery, perhaps involving microglial production of proinflammatory or anti-inflammatory cytokines and chemokines. Furthermore, alternatively activated M2-like microglia might promote phagocytosis of red blood cells and tissue debris, a major contribution to haematoma clearance. Interactions between microglia and other cells modulate microglial activation and function, and are also important in ICH pathology. This Review summarizes key studies on modulators of microglial activation and polarization after ICH, including M1-like and M2-like microglial phenotype markers, transcription factors and key signalling pathways. Microglial phagocytosis, haematoma resolution, and the potential crosstalk between microglia and T lymphocytes, neurons, astrocytes, and oligodendrocytes in the ICH brain are described. Finally, the clinical and translational implications of microglial polarization in ICH are presented, including the evidence that therapeutic approaches aimed at modulating microglial function might mitigate ICH injury and improve brain repair.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. - PubMed
-
- Steiner T, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9:840–855. - PubMed
-
- Thabet AM, Kottapally M, Hemphill JC., III Management of intracerebral hemorrhage. Handb Clin Neurol. 2017;140:177–194. - PubMed
-
- Hemphill JC, III , et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

