Polydatin reduces Staphylococcus aureus lipoteichoic acid-induced injury by attenuating reactive oxygen species generation and TLR2-NFκB signalling
- PMID: 28524642
- PMCID: PMC5661256
- DOI: 10.1111/jcmm.13194
Polydatin reduces Staphylococcus aureus lipoteichoic acid-induced injury by attenuating reactive oxygen species generation and TLR2-NFκB signalling
Abstract
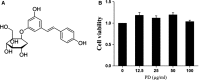
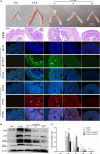
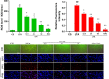
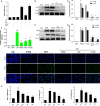
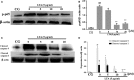
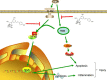
Staphylococcus aureus (S. aureus) causes severe inflammation in various infectious diseases, leading to high mortality. The clinical application of antibiotics has gained a significant curative effect. However, it has led to the emergence of various resistant bacteria. Therefore, in this study, we investigated the protective effect of polydatin (PD), a traditional Chinese medicine extract, on S. aureus lipoteichoic acid (LTA)-induced injury in vitro and in vivo. First, a significant improvement in the pathological conditions of PD in vivo was observed, suggesting that PD had a certain protective effect on LTA-induced injury in a mouse model. To further explore the underlying mechanisms of this protective effect of PD, LTA-induced murine macrophages were used in this study. The results have shown that PD could reduce the NF-κB p65, and IκBα phosphorylation levels increased by LTA, resulting in a decrease in the transcription of pro-inflammatory factors, such as TNF-α, IL-1β and IL-6. However, LTA can not only activate NF-κB through the recognition of TLR2 but also increase the level of intracellular reactive oxygen species (ROS), thereby activating NF-κB signalling. We also detected high levels of ROS that activate caspases 9 and 3 to induce apoptosis. In addition, using a specific NF-κB inhibitor that could attenuate apoptosis, namely NF-κB p65, acted as a pro-apoptotic transcription factor in LTA-induced murine macrophages. However, PD could inhibit the generation of ROS and NF-κB p65 activation, suggesting that PD suppressed LTA-induced injury by attenuating ROS generation and TLR2-NFκB signalling.
Keywords: ROS; NF-κB; apoptosis; inflammation.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

References
-
- Kriebs JM. Staphylococcus Infections in Pregnancy: maternal and Neonatal Risks. J Perinat Neonatal Nurs. 2016; 30: 115–23. - PubMed
-
- Johnston JN, Kaplan SL, Mason EO, et al Characterization of Staphylococcus aureus infections in children with down syndrome. J Infect Chemother. 2015; 21: 790–4. - PubMed
-
- Zhao G, Wu H, Jiang K, et al IFN‐τ inhibits S. aureus‐induced inflammation by suppressing the activation of NF‐κB and MAPKs in RAW 264.7 cells and mice with pneumonia. Int Immunopharmacol. 2016; 35: 332–40. - PubMed
-
- Seixas R, Varanda D, Bexiga R, et al Biofilm‐formation by Staphylococcus aureus and Staphylococcus epidermidis isolates from subclinical mastitis in conditions mimicking the udder environment. Pol J Vet Sci. 2015; 18: 787–92. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

