Sphingosine 1-phosphate signaling in bone remodeling: multifaceted roles and therapeutic potential
- PMID: 28524744
- PMCID: PMC5470107
- DOI: 10.1080/14728222.2017.1332180
Sphingosine 1-phosphate signaling in bone remodeling: multifaceted roles and therapeutic potential
Abstract
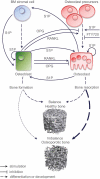
Sphingolipids belong to a complex class of lipid molecules that are crucially involved in the regulation of important biological processes including proliferation, migration and apoptosis. Given the significant progress made in understanding the sphingolipid pathobiology of several diseases, sphingolipid-related checkpoints emerge as attractive targets. Recent data indicate the multifaceted contribution of the sphingolipid machinery to osteoclast - osteoblast crosstalk, representing one of the pivotal interactions underlying bone homeostasis. Imbalances in the interplay of osteoblasts and osteoclasts might lead to bone-related diseases such as osteoporosis, rheumatoid arthritis, and bone metastases. Areas covered: We summarize and analyze the progress made in bone research in the context of the current knowledge of sphingolipid-related mechanisms regulating bone remodeling. Particular emphasis was given to bioactive sphingosine 1-phosphate (S1P) and S1P receptors (S1PRs). Moreover, the mechanisms of how dysregulations of this machinery cause bone diseases, are covered. Expert opinion: In the context of bone diseases, pharmacological interference with sphingolipid machinery may lead to novel directions in therapeutic strategies. Implementation of knowledge derived from in vivo animal models and in vitro studies using pharmacological agents to manipulate the S1P/S1PRs axes suggests S1PR2 and S1PR3 as potential drug targets, particularly in conjunction with technology for local drug delivery.
Keywords: Bone biology; bone diseases; coupling factor; osteoclast – osteoblast crosstalk; osteoporosis; osteotropic therapies; sphingolipid-related checkpoints; sphingosine 1-phosphate; sphingosine 1-phosphate receptor antagonist/agonist.
Figures


Similar articles
-
Role of Canonical and Non-Canonical Sphingolipids and their Metabolic Enzymes in Bone Health.Curr Osteoporos Rep. 2025 Apr 23;23(1):21. doi: 10.1007/s11914-025-00908-3. Curr Osteoporos Rep. 2025. PMID: 40266422 Free PMC article. Review.
-
Sphingosine-1-phosphate/S1PR2-mediated signaling triggers Smad1/5/8 phosphorylation and thereby induces Runx2 expression in osteoblasts.Bone. 2016 Dec;93:1-11. doi: 10.1016/j.bone.2016.09.003. Epub 2016 Sep 6. Bone. 2016. PMID: 27612439
-
Sphingosine-1-phosphate promotes osteogenesis by stimulating osteoblast growth and neovascularization in a vascular endothelial growth factor-dependent manner.J Bone Miner Res. 2024 Apr 19;39(3):357-372. doi: 10.1093/jbmr/zjae006. J Bone Miner Res. 2024. PMID: 38477738 Free PMC article.
-
The Species Effect: Differential Sphingosine-1-Phosphate Responses in the Bone in Human Versus Mouse.Int J Mol Sci. 2024 May 8;25(10):5118. doi: 10.3390/ijms25105118. Int J Mol Sci. 2024. PMID: 38791156 Free PMC article.
-
The role of sphingosine-1-phosphate (S1P) and lysophosphatidic acid (LPA) in regulation of osteoclastic and osteoblastic cells.Immunol Invest. 2013;42(7):510-8. doi: 10.3109/08820139.2013.823804. Immunol Invest. 2013. PMID: 24004055 Review.
Cited by
-
The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States.Front Cell Dev Biol. 2022 Feb 21;9:770143. doi: 10.3389/fcell.2021.770143. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35265628 Free PMC article. Review.
-
The Ying and Yang of Sphingosine-1-Phosphate Signalling within the Bone.Int J Mol Sci. 2023 Apr 8;24(8):6935. doi: 10.3390/ijms24086935. Int J Mol Sci. 2023. PMID: 37108099 Free PMC article. Review.
-
Role of Canonical and Non-Canonical Sphingolipids and their Metabolic Enzymes in Bone Health.Curr Osteoporos Rep. 2025 Apr 23;23(1):21. doi: 10.1007/s11914-025-00908-3. Curr Osteoporos Rep. 2025. PMID: 40266422 Free PMC article. Review.
-
Sphingosine-1-Phosphate Receptor 2 Controls Podosome Components Induced by RANKL Affecting Osteoclastogenesis and Bone Resorption.Cells. 2019 Jan 1;8(1):17. doi: 10.3390/cells8010017. Cells. 2019. PMID: 30609675 Free PMC article.
-
Multinucleated Giant Cells: Current Insights in Phenotype, Biological Activities, and Mechanism of Formation.Front Cell Dev Biol. 2022 Apr 11;10:873226. doi: 10.3389/fcell.2022.873226. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35478968 Free PMC article. Review.
References
-
- Rauner M, Sipos W, Pietschmann P. Osteoimmunology Int Arch Allergy Immunol. 2007;143(1):31–48. - PubMed
-
• This is a comprehensive review on osteoimmunology.
-
- Pacifici R. Osteoimmunology and its implications for transplantation. Am J Transplant. 2013. September;13(9):2245–2254. - PubMed
-
- Arron JR, Choi Y.. Bone versus immune system. Nature. 2000. November 30;408(6812):535–536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
