Ubiquitin enzymes in the regulation of immune responses
- PMID: 28524749
- PMCID: PMC5490640
- DOI: 10.1080/10409238.2017.1325829
Ubiquitin enzymes in the regulation of immune responses
Abstract
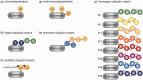
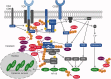
Ubiquitination plays a central role in the regulation of various biological functions including immune responses. Ubiquitination is induced by a cascade of enzymatic reactions by E1 ubiquitin activating enzyme, E2 ubiquitin conjugating enzyme, and E3 ubiquitin ligase, and reversed by deubiquitinases. Depending on the enzymes, specific linkage types of ubiquitin chains are generated or hydrolyzed. Because different linkage types of ubiquitin chains control the fate of the substrate, understanding the regulatory mechanisms of ubiquitin enzymes is central. In this review, we highlight the most recent knowledge of ubiquitination in the immune signaling cascades including the T cell and B cell signaling cascades as well as the TNF signaling cascade regulated by various ubiquitin enzymes. Furthermore, we highlight the TRIM ubiquitin ligase family as one of the examples of critical E3 ubiquitin ligases in the regulation of immune responses.
Keywords: B cell signaling; E3 ligase; T cell signaling; TNF signaling; TRIM; Ubiquitin; deubiquitinase.
Figures





Similar articles
-
Targeting the Ubiquitin Signaling Cascade in Tumor Microenvironment for Cancer Therapy.Int J Mol Sci. 2021 Jan 14;22(2):791. doi: 10.3390/ijms22020791. Int J Mol Sci. 2021. PMID: 33466790 Free PMC article. Review.
-
Dual-color pulse-chase ubiquitination assays to simultaneously monitor substrate priming and extension.Methods Enzymol. 2019;618:29-48. doi: 10.1016/bs.mie.2019.01.004. Epub 2019 Feb 11. Methods Enzymol. 2019. PMID: 30850057
-
Identification and Characterization of Physiological Pairing of E2 Ubiquitin-Conjugating Enzymes and E3 Ubiquitin Ligases.Methods Mol Biol. 2023;2581:13-29. doi: 10.1007/978-1-0716-2784-6_2. Methods Mol Biol. 2023. PMID: 36413307
-
Enzymes of ubiquitination and deubiquitination.Essays Biochem. 2012;52:37-50. doi: 10.1042/bse0520037. Essays Biochem. 2012. PMID: 22708562 Review.
-
New Insights into the Role of Ubiquitin Networks in the Regulation of Antiapoptosis Pathways.Int Rev Cell Mol Biol. 2015;318:121-58. doi: 10.1016/bs.ircmb.2015.05.003. Epub 2015 Jun 17. Int Rev Cell Mol Biol. 2015. PMID: 26315885 Review.
Cited by
-
Changes in expression of interferon-stimulated genes and ubiquitin activating enzyme E1-like in ovine thymus during early pregnancy.Anim Reprod. 2020 Jun 29;17(2):e20190134. doi: 10.1590/1984-3143-AR2019-0134. Anim Reprod. 2020. PMID: 32714456 Free PMC article.
-
MicroRNA-193a-5p Rescues Ischemic Cerebral Injury by Restoring N2-Like Neutrophil Subsets.Transl Stroke Res. 2023 Aug;14(4):589-607. doi: 10.1007/s12975-022-01071-y. Epub 2022 Jul 29. Transl Stroke Res. 2023. PMID: 35906328 Free PMC article.
-
Quantitative Analysis of Ubiquitinated Proteins in Human Pituitary and Pituitary Adenoma Tissues.Front Endocrinol (Lausanne). 2019 May 22;10:328. doi: 10.3389/fendo.2019.00328. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31191455 Free PMC article.
-
Molecular Characterization and Expression Analysis of ftr01, ftr42, and ftr58 in Zebrafish (Danio rerio).Virol Sin. 2019 Aug;34(4):434-443. doi: 10.1007/s12250-019-00112-5. Epub 2019 Apr 15. Virol Sin. 2019. PMID: 30989427 Free PMC article.
-
The Two Deubiquitinating Enzymes from Chlamydia trachomatis Have Distinct Ubiquitin Recognition Properties.Biochemistry. 2020 Apr 28;59(16):1604-1617. doi: 10.1021/acs.biochem.9b01107. Epub 2020 Apr 14. Biochemistry. 2020. PMID: 32275137 Free PMC article.
References
-
- Abramson J. and Goldfarb Y., 2016. AIRE: from promiscuous molecular partnerships to promiscuous gene expression. European journal of immunology, 46, 22–33. - PubMed
-
- Aggarwal B.B., 2003. Signalling pathways of the TNF superfamily: a double-edged sword. Nature reviews immunology, 3, 745–756. - PubMed
-
- Aireakiyama T., et al, 2008. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity, 29, 423–437. - PubMed
-
- Akiyama T., et al, 2005. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science, 308, 248–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
