Surface chemistry governs cellular tropism of nanoparticles in the brain
- PMID: 28524852
- PMCID: PMC5454541
- DOI: 10.1038/ncomms15322
Surface chemistry governs cellular tropism of nanoparticles in the brain
Abstract
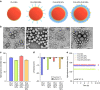
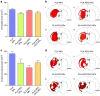
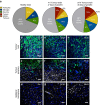
Nanoparticles are of long-standing interest for the treatment of neurological diseases such as glioblastoma. Most past work focused on methods to introduce nanoparticles into the brain, suggesting that reaching the brain interstitium will be sufficient to ensure therapeutic efficacy. However, optimized nanoparticle design for drug delivery to the central nervous system is limited by our understanding of their cellular deposition in the brain. Here, we investigated the cellular fate of poly(lactic acid) nanoparticles presenting different surface chemistries, after administration by convection-enhanced delivery. We demonstrate that nanoparticles with 'stealth' properties mostly avoid internalization by all cell types, but internalization can be enhanced by functionalization with bio-adhesive end-groups. We also show that association rates measured in cultured cells predict the extent of internalization of nanoparticles in cell populations. Finally, evaluating therapeutic efficacy in an orthotopic model of glioblastoma highlights the need to balance significant uptake without inducing adverse toxicity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
Biodegradable polymeric nanoparticles administered in the cerebrospinal fluid: Brain biodistribution, preferential internalization in microglia and implications for cell-selective drug release.Biomaterials. 2019 Jul;209:25-40. doi: 10.1016/j.biomaterials.2019.04.012. Epub 2019 Apr 14. Biomaterials. 2019. PMID: 31026609
-
Quantification of cellular and nuclear uptake rates of polymeric gene delivery nanoparticles and DNA plasmids via flow cytometry.Acta Biomater. 2016 Jun;37:120-30. doi: 10.1016/j.actbio.2016.03.036. Epub 2016 Mar 24. Acta Biomater. 2016. PMID: 27019146 Free PMC article.
-
Convection-Enhanced Delivery of Carboplatin PLGA Nanoparticles for the Treatment of Glioblastoma.PLoS One. 2015 Jul 17;10(7):e0132266. doi: 10.1371/journal.pone.0132266. eCollection 2015. PLoS One. 2015. PMID: 26186224 Free PMC article.
-
Nanocarriers for the treatment of glioblastoma multiforme: Current state-of-the-art.J Control Release. 2016 Apr 10;227:23-37. doi: 10.1016/j.jconrel.2016.02.026. Epub 2016 Feb 16. J Control Release. 2016. PMID: 26892752 Review.
-
Overview of the preparation of organic polymeric nanoparticles for drug delivery based on gelatine, chitosan, poly(d,l-lactide-co-glycolic acid) and polyalkylcyanoacrylate.Colloids Surf B Biointerfaces. 2014 Jun 1;118:154-63. doi: 10.1016/j.colsurfb.2014.03.017. Epub 2014 Apr 1. Colloids Surf B Biointerfaces. 2014. PMID: 24769392 Review.
Cited by
-
Novel multi-drugs incorporating hybrid-structured nanofibers enhance alkylating agent activity in malignant gliomas.Ther Adv Med Oncol. 2019 Sep 26;11:1758835919875555. doi: 10.1177/1758835919875555. eCollection 2019. Ther Adv Med Oncol. 2019. PMID: 31632467 Free PMC article.
-
Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics.Biomedicines. 2020 Jan 13;8(1):13. doi: 10.3390/biomedicines8010013. Biomedicines. 2020. PMID: 31941057 Free PMC article. Review.
-
Nonsurgical treatment of skin cancer with local delivery of bioadhesive nanoparticles.Proc Natl Acad Sci U S A. 2021 Feb 16;118(7):e2020575118. doi: 10.1073/pnas.2020575118. Proc Natl Acad Sci U S A. 2021. PMID: 33526595 Free PMC article.
-
Biodegradable PEG-poly(ω-pentadecalactone-co-p-dioxanone) nanoparticles for enhanced and sustained drug delivery to treat brain tumors.Biomaterials. 2018 Sep;178:193-203. doi: 10.1016/j.biomaterials.2018.06.024. Epub 2018 Jun 18. Biomaterials. 2018. PMID: 29936153 Free PMC article.
-
Surface Presentation of Hyaluronic Acid Modulates Nanoparticle-Cell Association.Bioconjug Chem. 2022 Nov 16;33(11):2065-2075. doi: 10.1021/acs.bioconjchem.2c00412. Epub 2022 Oct 25. Bioconjug Chem. 2022. PMID: 36282941 Free PMC article.
References
-
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer 5, 161–171 (2005). - PubMed
-
- Wen P. Y. & Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 359, 492–507 (2008). - PubMed
-
- Gao H., Pang Z. & Jiang X. Targeted delivery of nano-therapeutics for major disorders of the central nervous system. Pharm. Res. 30, 2485–2498 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

