Targeted Degradation of CTCF Decouples Local Insulation of Chromosome Domains from Genomic Compartmentalization
- PMID: 28525758
- PMCID: PMC5538188
- DOI: 10.1016/j.cell.2017.05.004
Targeted Degradation of CTCF Decouples Local Insulation of Chromosome Domains from Genomic Compartmentalization
Abstract
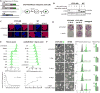
The molecular mechanisms underlying folding of mammalian chromosomes remain poorly understood. The transcription factor CTCF is a candidate regulator of chromosomal structure. Using the auxin-inducible degron system in mouse embryonic stem cells, we show that CTCF is absolutely and dose-dependently required for looping between CTCF target sites and insulation of topologically associating domains (TADs). Restoring CTCF reinstates proper architecture on altered chromosomes, indicating a powerful instructive function for CTCF in chromatin folding. CTCF remains essential for TAD organization in non-dividing cells. Surprisingly, active and inactive genome compartments remain properly segregated upon CTCF depletion, revealing that compartmentalization of mammalian chromosomes emerges independently of proper insulation of TADs. Furthermore, our data support that CTCF mediates transcriptional insulator function through enhancer blocking but not as a direct barrier to heterochromatin spreading. Beyond defining the functions of CTCF in chromosome folding, these results provide new fundamental insights into the rules governing mammalian genome organization.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


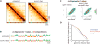



References
-
- Bickmore WA, van Steensel B. Genome Architecture: Domain Organization of Interphase Chromosomes. Cell. 2013;152:1270–1284. - PubMed
References for the Methods Sectons
MeSH terms
Substances
Grants and funding
- U01 HL098179/HL/NHLBI NIH HHS/United States
- R01 GM112720/GM/NIGMS NIH HHS/United States
- R01 GM114190/GM/NIGMS NIH HHS/United States
- U01 HL131003/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI117839/AI/NIAID NIH HHS/United States
- U54 HG007010/HG/NHGRI NIH HHS/United States
- U54 CA193419/CA/NCI NIH HHS/United States
- R01 HG003143/HG/NHGRI NIH HHS/United States
- U01 HG007910/HG/NHGRI NIH HHS/United States
- UM1 HL098179/HL/NHLBI NIH HHS/United States
- U54 DK107980/DK/NIDDK NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- U01 DA040588/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

