BAlloon versus Stenting in severe Ischaemia of the Leg-3 (BASIL-3): study protocol for a randomised controlled trial
- PMID: 28526046
- PMCID: PMC5438558
- DOI: 10.1186/s13063-017-1968-6
BAlloon versus Stenting in severe Ischaemia of the Leg-3 (BASIL-3): study protocol for a randomised controlled trial
Abstract
Background: Severe limb ischaemia (SLI) is defined as the presence of rest pain and/or tissue loss secondary to lower extremity atherosclerotic peripheral arterial disease. The superficial femoral and popliteal arteries are the most commonly diseased vessels in such patients and are being increasingly treated using endovascular revascularisation techniques. However, it is currently unknown whether drug-eluting stents and drug-coated balloons confer additional clinical benefits over more established techniques using plain balloons and bare metal stents, or whether they represent a cost-effective use of NHS resources.
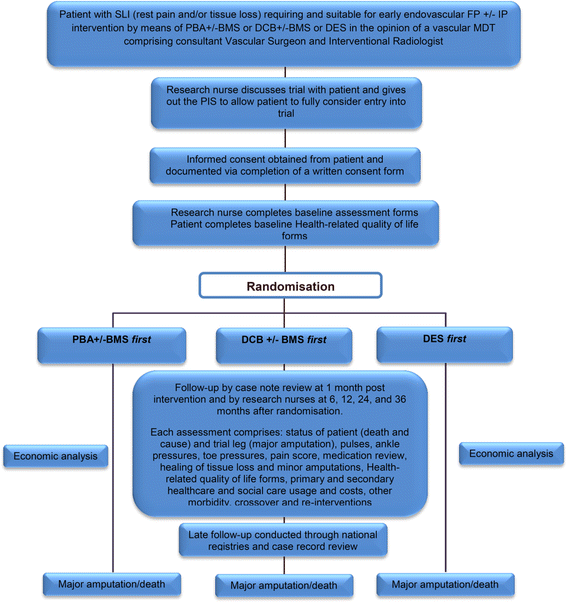
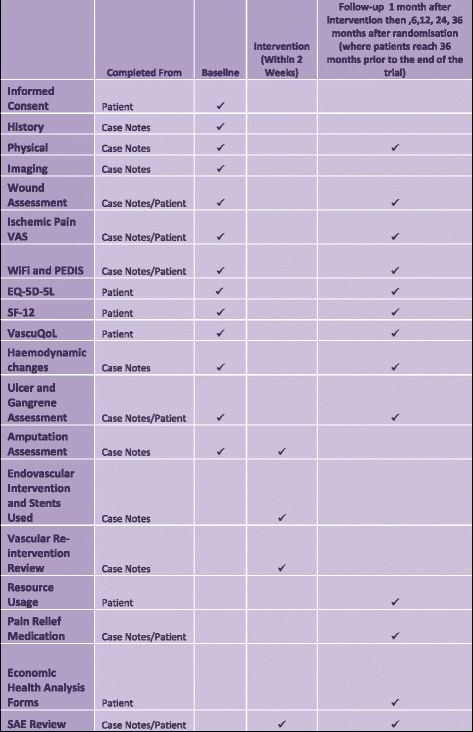
Methods: The BASIL-3 trial is a UK National Institute for Health Research, Health Technology Assessment Programme-funded, multicentre, randomised controlled trial (RCT) comparing the clinical and cost-effectiveness of plain balloon angioplasty with or without bail-out bare metal stenting, drug-coated balloon angioplasty with or without bail-out bare metal stenting, and primary stenting with drug-eluting stents for SLI secondary to femoro-popliteal disease. Patients with 'multilevel' disease may receive aorto-iliac and/or infrapopliteal treatments concurrently with their randomised femoro-popliteal intervention. The primary clinical outcome is amputation-free survival defined as the time to major (above the ankle) amputation of the index limb or death from any cause. The primary outcome for the economic analysis is cost per quality-adjusted life year. Secondary outcome measures include overall survival, major adverse limb events, major adverse cardiac events, relief of ischaemic pain, healing of tissue loss, and quality of life. The required sample size has been calculated at 861 participants (287 on each arm). These patients will be recruited over 3 years and followed-up for between 2 and 5 years.
Discussion: BASIL-3 is a pragmatic RCT designed to reflect current UK clinical practice. The results will inform decision-making regarding the appropriateness of funding the use of drug-coated balloons and drug-eluting stents, by the NHS, for the management of SLI due to femoro-popliteal disease.
Trial registration: ISRCTN Registry, identifier: ISRCTN14469736 . Registered on 22 October 2015.
Keywords: Angioplasty; Cost-effectiveness; Critical limb ischaemia; Diabetes; Drug-coated balloon; Drug-eluting stent; Endovascular treatment; Severe limb ischaemia; Stent.
Figures
Similar articles
-
Plain versus drug balloon and stenting in severe ischaemia of the leg (BASIL-3): open label, three arm, randomised, multicentre, phase 3 trial.BMJ. 2025 Feb 24;388:e080881. doi: 10.1136/bmj-2024-080881. BMJ. 2025. PMID: 39993822 Free PMC article. Clinical Trial.
-
Vein bypass first vs. best endovascular treatment first revascularisation strategy for chronic limb-threatening ischaemia due to infra-popliteal disease: the BASIL-2 RCT.Health Technol Assess. 2024 Oct;28(65):1-72. doi: 10.3310/YTFV4524. Health Technol Assess. 2024. PMID: 39397484 Free PMC article. Clinical Trial.
-
Comparative Effectiveness of Plain Balloon Angioplasty, Bare Metal Stents, Drug-Coated Balloons, and Drug-Eluting Stents for the Treatment of Infrapopliteal Artery Disease: Systematic Review and Bayesian Network Meta-analysis of Randomized Controlled Trials.J Endovasc Ther. 2016 Dec;23(6):851-863. doi: 10.1177/1526602816671740. Epub 2016 Oct 5. J Endovasc Ther. 2016. PMID: 27708143
-
Long-Term Follow-up of the PADI Trial: Percutaneous Transluminal Angioplasty Versus Drug-Eluting Stents for Infrapopliteal Lesions in Critical Limb Ischemia.J Am Heart Assoc. 2017 Apr 14;6(4):e004877. doi: 10.1161/JAHA.116.004877. J Am Heart Assoc. 2017. PMID: 28411244 Free PMC article. Clinical Trial.
-
Economic analysis of endovascular interventions for femoropopliteal arterial disease: a systematic review and budget impact model for the United States and Germany.Catheter Cardiovasc Interv. 2014 Oct 1;84(4):546-54. doi: 10.1002/ccd.25536. Epub 2014 May 27. Catheter Cardiovasc Interv. 2014. PMID: 24782424
Cited by
-
Ambulatory Status Over Time after Revascularization in Patients with Chronic Limb-Threatening Ischemia.J Atheroscler Thromb. 2022 Jun 1;29(6):866-880. doi: 10.5551/jat.62892. Epub 2021 May 27. J Atheroscler Thromb. 2022. PMID: 34039832 Free PMC article.
-
Paclitaxel-Coated Balloons and Stents for Lower Extremity Peripheral Arterial Disease Interventions: A Regulatory Perspective for the Practicing Clinician.Mayo Clin Proc. 2020 Aug;95(8):1569-1573. doi: 10.1016/j.mayocp.2020.01.035. Mayo Clin Proc. 2020. PMID: 32753129 Free PMC article. No abstract available.
-
Comprehensive Assessment of Current Management Strategies for Patients With Diabetes and Chronic Limb-Threatening Ischemia.Clin Diabetes. 2021 Oct;39(4):358-388. doi: 10.2337/cd21-0019. Clin Diabetes. 2021. PMID: 34866779 Free PMC article.
-
Outcomes After Drug-Coated Balloon Treatment of Femoropopliteal Lesions in Patients With Critical Limb Ischemia: A Post Hoc Analysis From the IN.PACT Global Study.J Endovasc Ther. 2019 Jun;26(3):305-315. doi: 10.1177/1526602819839044. Epub 2019 Apr 1. J Endovasc Ther. 2019. PMID: 30931726 Free PMC article.
-
Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia.Eur J Vasc Endovasc Surg. 2019 Jul;58(1S):S1-S109.e33. doi: 10.1016/j.ejvs.2019.05.006. Epub 2019 Jun 8. Eur J Vasc Endovasc Surg. 2019. PMID: 31182334 Free PMC article.
References
-
- National Institute for Health and Clinical Excellence. Lower limb peripheral arterial disease. Diagnosis and management. NICE clinical guideline 147. In: National Clinical Guideline Centre. 2012.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical