Testing times: trends in availability, price, and market share of malaria diagnostics in the public and private healthcare sector across eight sub-Saharan African countries from 2009 to 2015
- PMID: 28526075
- PMCID: PMC5438573
- DOI: 10.1186/s12936-017-1829-5
Testing times: trends in availability, price, and market share of malaria diagnostics in the public and private healthcare sector across eight sub-Saharan African countries from 2009 to 2015
Abstract
Background: The World Health Organization guidelines have recommended that all cases of suspected malaria should receive a confirmatory test with microscopy or a malaria rapid diagnostic test (RDT), however evidence from sub-Saharan Africa (SSA) illustrates that only one-third of children under five with a recent fever received a test. The aim of this study was to evaluate availability, price and market share of microscopy and RDT from 2009/11 to 2014/15 in 8 SSA countries, to better understand barriers to improving access to malaria confirmatory testing in the public and private health sectors.
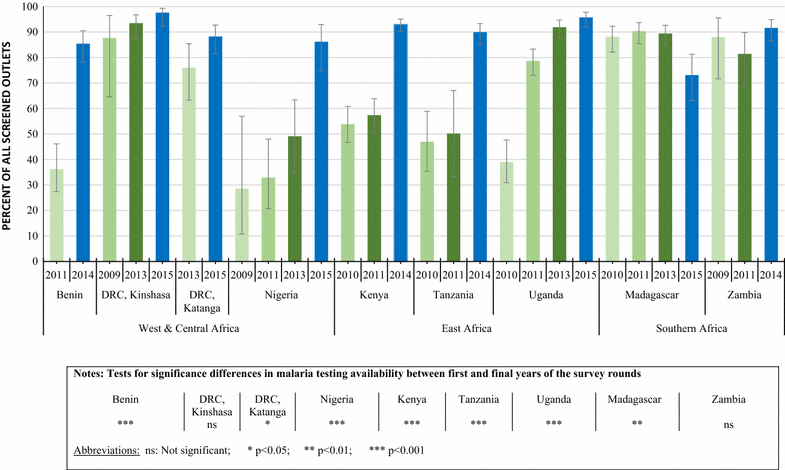
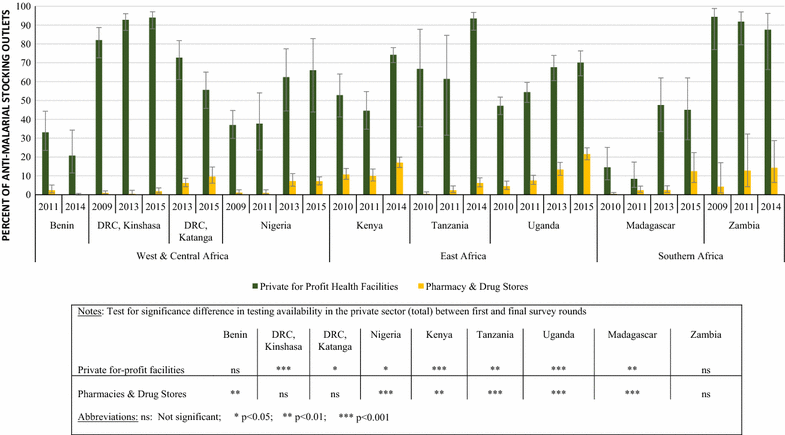
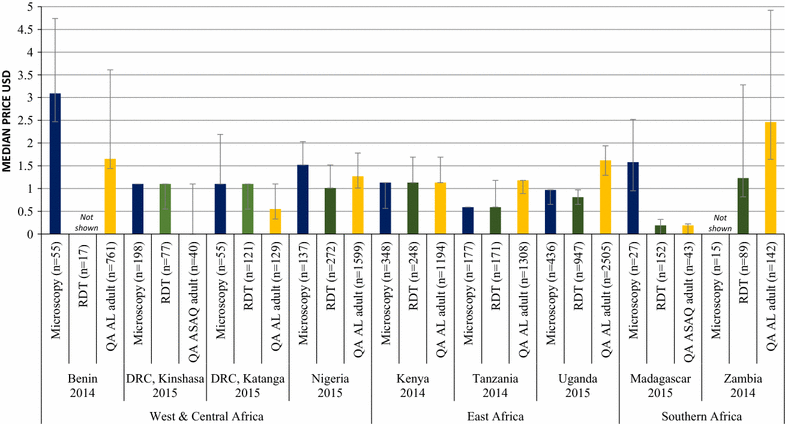
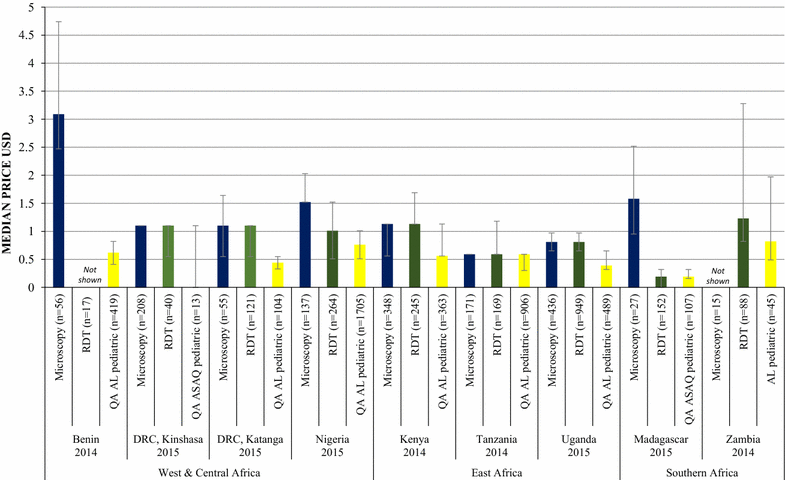
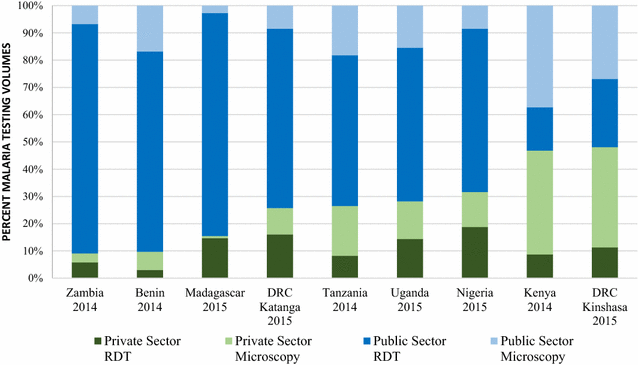
Results: Repeated national cross-sectional quantitative surveys were conducted among a sample of outlets stocking anti-malarial medicines and/or diagnostics. In total, 169,655 outlets were screened. Availability of malaria blood testing among all screened public health facilities increased significantly between the first survey wave in 2009/11 and the most recent in 2014/15 in Benin (36.2, 85.4%, p < 0.001), Kenya (53.8, 93.0%, p < 0.001), mainland Tanzania (46.9, 89.9%, p < 0.001), Nigeria (28.5, 86.2%, p < 0.001), Katanga, the Democratic Republic of the Congo (DRC) (76.0, 88.2%, p < 0.05), and Uganda (38.9, 95.6%, p < 0.001). These findings were attributed to an increase in availability of RDTs. Diagnostic availability remained high in Kinshasa (the DRC) (87.6, 97.6%) and Zambia (87.9, 91.6%). Testing availability in public health facilities significantly decreased in Madagascar (88.1, 73.1%, p < 0.01). In the most recent survey round, the majority of malaria testing was performed in the public sector in Zambia (90.9%), Benin (90.3%), Madagascar (84.5%), Katanga (74.3%), mainland Tanzania (73.5%), Uganda (71.8%), Nigeria (68.4%), Kenya (53.2%) and Kinshasa (51.9%). In the anti-malarial stocking private sector, significant increases in availability of diagnostic tests among private for-profit facilities were observed between the first and final survey rounds in Kinshasa (82.1, 94.0%, p < 0.05), Nigeria (37.0, 66.0%, p < 0.05), Kenya (52.8, 74.3%, p < 0.001), mainland Tanzania (66.8, 93.5%, p < 0.01), Uganda (47.1, 70.1%, p < 0.001), and Madagascar (14.5, 45.0%, p < 0.01). Blood testing availability remained low over time among anti-malarial stocking private health facilities in Benin (33.1, 20.7%), and high over time in Zambia (94.4, 87.5%), with evidence of falls in availability in Katanga (72.7, 55.6%, p < 0.05). Availability among anti-malarial stocking pharmacies and drug stores-which are the most common source of anti-malarial medicines-was rare in all settings, and highest in Uganda in 2015 (21.5%). Median private sector price of RDT for a child was equal to the price of pre-packaged quality-assured artemisinin-based combination therapy (QAACT) treatment for a two-year old child in some countries, and 1.5-2.5 times higher in others. Median private sector QAACT price for an adult varied from having parity with an RDT for an adult to being up to 2 times more expensive. The exception was in both Kinshasa and Katanga, where the median price of QAACT was less expensive than RDTs.
Conclusions: Significant strides have been made in the availability of testing, mainly through the widespread distribution of RDT, and especially in public health facilities. Significant barriers to universal coverage of diagnostic testing can be attributed to very low availability in the private sector, particularly among pharmacies and drug stores, which are responsible for most anti-malarial distribution. Where tests are available, price may serve as a barrier to uptake, particularly for young children. Several initiatives that have introduced RDT into the private sector can be modified and expanded as a means to close this gap in malaria testing availability and promote universal diagnosis.
Keywords: Availability; Malaria test; Market share; Microscopy; Price; Rapid diagnostic test; sub-Saharan Africa.
Figures
Similar articles
-
What happened to anti-malarial markets after the Affordable Medicines Facility-malaria pilot? Trends in ACT availability, price and market share from five African countries under continuation of the private sector co-payment mechanism.Malar J. 2017 Apr 25;16(1):173. doi: 10.1186/s12936-017-1814-z. Malar J. 2017. PMID: 28441956 Free PMC article.
-
Availability and price of malaria rapid diagnostic tests in the public and private health sectors in 2011: results from 10 nationally representative cross-sectional retail surveys.Trop Med Int Health. 2015 Jun;20(6):744-56. doi: 10.1111/tmi.12491. Epub 2015 Mar 22. Trop Med Int Health. 2015. PMID: 25728761
-
The malaria testing and treatment market in Kinshasa, Democratic Republic of the Congo, 2013.Malar J. 2017 Feb 28;16(1):94. doi: 10.1186/s12936-016-1659-x. Malar J. 2017. PMID: 28241832 Free PMC article.
-
Private sector opportunities and threats to achieving malaria elimination in the Greater Mekong Subregion: results from malaria outlet surveys in Cambodia, the Lao PDR, Myanmar, and Thailand.Malar J. 2017 May 2;16(1):180. doi: 10.1186/s12936-017-1800-5. Malar J. 2017. PMID: 28464945 Free PMC article. Review.
-
Diagnosis and Treatment of the Febrile Child.In: Black RE, Laxminarayan R, Temmerman M, Walker N, editors. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2016 Apr 5. Chapter 8. In: Black RE, Laxminarayan R, Temmerman M, Walker N, editors. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2016 Apr 5. Chapter 8. PMID: 27227231 Free Books & Documents. Review.
Cited by
-
Measuring malaria diagnosis and treatment coverage in population-based surveys: a recall validation study in Mali among caregivers of febrile children under 5 years.Malar J. 2019 Jan 3;18(1):3. doi: 10.1186/s12936-018-2636-3. Malar J. 2019. PMID: 30602376 Free PMC article.
-
Addressing antimicrobial resistance by improving access and quality of care-A review of the literature from East Africa.PLoS Negl Trop Dis. 2021 Jul 22;15(7):e0009529. doi: 10.1371/journal.pntd.0009529. eCollection 2021 Jul. PLoS Negl Trop Dis. 2021. PMID: 34292932 Free PMC article. Review.
-
Improving malaria case management with artemisinin-based combination therapies and malaria rapid diagnostic tests in private medicine retail outlets in sub-Saharan Africa: A systematic review.PLoS One. 2024 Jul 29;19(7):e0286718. doi: 10.1371/journal.pone.0286718. eCollection 2024. PLoS One. 2024. PMID: 39074113 Free PMC article.
-
Aetiology of non-malaria acute febrile illness fever in children in rural Guinea-Bissau: a prospective cross-sectional investigation.Front Epidemiol. 2024 Mar 21;4:1309149. doi: 10.3389/fepid.2024.1309149. eCollection 2024. Front Epidemiol. 2024. PMID: 38577653 Free PMC article.
-
Management of childhood pneumonia in Nigeria.Pediatr Pulmonol. 2020 Jun;55 Suppl 1(Suppl 1):S34-S36. doi: 10.1002/ppul.24717. Epub 2020 Mar 23. Pediatr Pulmonol. 2020. PMID: 32203644 Free PMC article. No abstract available.
References
-
- WHO. World Malaria Report. Geneva: World Health Organization; 2016. http://www.who.int/malaria/publications/world-malaria-report-2015/report.... Accessed 04 Mar 2017.
-
- WHO. T3: Test. Treat. Track. Scaling up diagnostic testing, treatment and surveillance for malaria. Geneva: World Health Organization; 2016. http://www.who.int/malaria/publications/atoz/test_treat_track_brochure.p.... Accessed 04 Mar 2017.
-
- Yukich JO, Bennett A, Albertini A, Incardona S, Moonga H, Chisha Z, et al. Reductions in artemisinin-based combination therapy consumption after the nationwide scale up of routine malaria rapid diagnostic testing in Zambia. Am J Trop Med Hyg. 2012;87:437–446. doi: 10.4269/ajtmh.2012.12-0127. - DOI - PMC - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

