GBT1118, a potent allosteric modifier of hemoglobin O2 affinity, increases tolerance to severe hypoxia in mice
- PMID: 28526710
- PMCID: PMC5582925
- DOI: 10.1152/ajpheart.00772.2016
GBT1118, a potent allosteric modifier of hemoglobin O2 affinity, increases tolerance to severe hypoxia in mice
Abstract
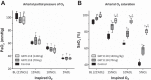
Adaptation to hypoxia requires compensatory mechanisms that affect O2 transport and utilization. Decreased hemoglobin (Hb) O2 affinity is considered part of the physiological adaptive process to chronic hypoxia. However, this study explores the hypothesis that increased Hb O2 affinity can complement acute physiological responses to hypoxia by increasing O2 uptake and delivery compared with normal Hb O2 affinity during acute severe hypoxia. To test this hypothesis, Hb O2 affinity in mice was increased by oral administration of 2-hydroxy-6-{[(2S)-1-(pyridine-3-carbonyl)piperidin-2yl] methoxy}benzaldehyde (GBT1118; 70 or 140 mg/kg). Systemic and microcirculatory hemodynamics and oxygenation parameters were studied during hypoxia in awake-instrumented mice. GBT1118 increased Hb O2 affinity and decreased the Po2 at which 50% of Hb is saturated with O2 (P50) from 43 ± 1.1 to 18.3 ± 0.9 mmHg (70 mg/kg) and 7.7 ± 0.2 mmHg (140 mg/kg). In a dose-dependent fashion, GBT1118 increased arterial O2 saturation by 16% (70 mg/kg) and 40% (140 mg/kg) relative to the control group during 5% O2 hypoxia. In addition, a GBT1118-induced increase in Hb O2 affinity reduced hypoxia-induced hypotension compared with the control group. Moreover, microvascular blood flow was higher during hypoxia in GBT1118-treated groups than the control group. The increased O2 saturation and improved blood flow in GBT1118-treated groups preserved higher interstitial tissue Po2 than in the control group during 5% O2 hypoxia. In conclusion, increased Hb O2 affinity enhanced physiological tolerance to hypoxia, as evidenced by improved hemodynamics and tissue oxygenation. Therefore, pharmacologically induced increases in Hb O2 affinity become a potential therapeutic approach to improve tissue oxygenation in pulmonary diseases characterized by severe hypoxemia.NEW & NOTEWORTHY This study establishes that pharmacological modification of hemoglobin O2 affinity can be a promising and novel therapeutic strategy for the treatment of hypoxic hypoxia and paves the way for the clinical development of molecules that prevent hypoxemia.
Keywords: hemoglobin; hypoxia; microcirculation; oxygen; oxygen delivery; partial pressure of oxygen at which hemoglobin is 50% saturated with oxygen; tissue partial pressure of oxygen.
Copyright © 2017 the American Physiological Society.
Conflict of interest statement
K. Dufu, A. Hutchaleelala, Q. Xu, Z. Li, N. Vlahakis, D. Oksenberg, and J. Lehrer-Graiwer are employees and shareholders of Global Blood Therapeutics Incorporated. All other authors declare no competing financial interests by the results presented in this report. Financial support was received from Global Blood Therapeutics Incorporated for the completion of the study. Global Blood Therapeutics Incorporated did not participate in the implementation of the experimental study.
Figures






Similar articles
-
Increased hemoglobin affinity for oxygen with GBT1118 improves hypoxia tolerance in sickle cell mice.Am J Physiol Heart Circ Physiol. 2021 Aug 1;321(2):H400-H411. doi: 10.1152/ajpheart.00048.2021. Epub 2021 Jul 2. Am J Physiol Heart Circ Physiol. 2021. PMID: 34213392 Free PMC article.
-
Increased hemoglobin-oxygen affinity ameliorates bleomycin-induced hypoxemia and pulmonary fibrosis.Physiol Rep. 2016 Sep;4(17):e12965. doi: 10.14814/phy2.12965. Physiol Rep. 2016. PMID: 27624688 Free PMC article.
-
GBT1118, a compound that increases the oxygen affinity of hemoglobin, improves survival in murine hypoxic acute lung injury.J Appl Physiol (1985). 2018 Apr 1;124(4):899-905. doi: 10.1152/japplphysiol.00079.2017. Epub 2017 Dec 14. J Appl Physiol (1985). 2018. PMID: 29357510 Free PMC article.
-
Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle.Circ Res. 2020 Jan 3;126(1):129-158. doi: 10.1161/CIRCRESAHA.119.315626. Epub 2019 Oct 8. Circ Res. 2020. PMID: 31590598 Free PMC article. Review.
-
Hemoglobin-oxygen affinity in high-altitude vertebrates: is there evidence for an adaptive trend?J Exp Biol. 2016 Oct 15;219(Pt 20):3190-3203. doi: 10.1242/jeb.127134. J Exp Biol. 2016. PMID: 27802149 Free PMC article. Review.
Cited by
-
Time Domains of Hypoxia Responses and -Omics Insights.Front Physiol. 2022 Aug 8;13:885295. doi: 10.3389/fphys.2022.885295. eCollection 2022. Front Physiol. 2022. PMID: 36035495 Free PMC article. Review.
-
GBT1118, a voxelotor analog, protects red blood cells from damage during severe hypoxia.Am J Transl Res. 2022 Jan 15;14(1):240-251. eCollection 2022. Am J Transl Res. 2022. PMID: 35173841 Free PMC article.
-
Rheological Impact of GBT1118 Cessation in a Sickle Mouse Model.Front Physiol. 2021 Sep 24;12:742784. doi: 10.3389/fphys.2021.742784. eCollection 2021. Front Physiol. 2021. PMID: 34630162 Free PMC article.
-
[Research progress on mechanism in adaptation of hemoglobin to plateau hypoxia].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019 Dec 25;48(6):674-681. doi: 10.3785/j.issn.1008-9292.2019.12.13. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019. PMID: 31955543 Free PMC article. Review. Chinese.
-
Statistical considerations in reporting cardiovascular research.Am J Physiol Heart Circ Physiol. 2018 Aug 1;315(2):H303-H313. doi: 10.1152/ajpheart.00309.2018. Epub 2018 Jul 20. Am J Physiol Heart Circ Physiol. 2018. PMID: 30028200 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

