A broad-spectrum bactericidal lipopeptide with anti-biofilm properties
- PMID: 28526864
- PMCID: PMC5438364
- DOI: 10.1038/s41598-017-02373-0
A broad-spectrum bactericidal lipopeptide with anti-biofilm properties
Abstract
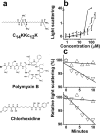
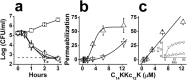
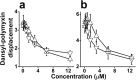
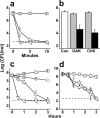
Previous studies of the oligoacyllysyl (OAK) series acyl-lysyl-lysyl-aminoacyl-lysine-amide, suggested their utility towards generating robust linear lipopeptide-like alternatives to antibiotics, although to date, none exhibited potent broad-spectrum bactericidal activity. To follow up on this premise, we produced a new analog (C14KKc12K) and investigated its properties in various media. Mechanistic studies suggest that C14KKc12K uses a non-specific membrane-disruptive mode of action for rapidly reducing viability of Gram-negative bacteria (GNB) similarly to polymyxin B (PMB), a cyclic lipopeptide used as last resort antibiotic. Indeed, C14KKc12K displayed similar affinity for lipopolysaccharides and induced cell permeabilization associated with rapid massive membrane depolarization. Unlike PMB however, C14KKc12K was also bactericidal to Gram-positive bacteria (GPB) at or near the minimal inhibitory concentration (MIC), as assessed against a multispecies panel of >50 strains, displaying MIC50 at 3 and 6 µM, respectively for GPB and GNB. C14KKc12K retained activity in human saliva, reducing the viability of cultivable oral microflora by >99% within two minutes of exposure, albeit at higher concentrations, which, nonetheless, were similar to the commercial gold standard, chlorhexidine. This equipotent bactericidal activity was also observed in pre-formed biofilms of Streptococcus mutans, a major periodontal pathogen. Such compounds therefore, may be useful for eradication of challenging poly-microbial infections.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

