EndophilinAs regulate endosomal sorting of BDNF-TrkB to mediate survival signaling in hippocampal neurons
- PMID: 28526875
- PMCID: PMC5438371
- DOI: 10.1038/s41598-017-02202-4
EndophilinAs regulate endosomal sorting of BDNF-TrkB to mediate survival signaling in hippocampal neurons
Abstract
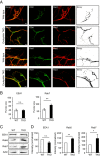
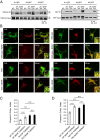
The sorting of activated receptors into distinct endosomal compartments is essential to activate specific signaling cascades and cellular events including growth and survival. However, the proteins involved in this sorting are not well understood. We discovered a novel role of EndophilinAs in sorting of activated BDNF-TrkB receptors into late endosomal compartments. Mice lacking all three EndophilinAs accumulate Rab7-positive late endosomes. Moreover, EndophilinAs are differentially localized to, co-traffic with, and tubulate, distinct endosomal compartments: In response to BDNF, EndophilinA2 is recruited to both early and late endosomes, EndophilinA3 is recruited to Lamp1-positive late endosomes, and co-trafficks with Rab5 and Rab7 in both the presence and absence of BDNF, while EndophilinA1 colocalizes at lower levels with endosomes. The absence of all three EndophilinAs caused TrkB to accumulate in EEA1 and Rab7-positive endosomes, and impaired BDNF-TrkB-dependent survival signaling cascades. In addition, EndophilinA triple knockout neurons exhibited increased cell death which could not be rescued by exogenous BDNF, in a neurotrophin-dependent survival assay. Thus, EndophilinAs differentially regulate activated receptor sorting via distinct endosomal compartments to promote BDNF-dependent cell survival.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Retrolinkin cooperates with endophilin A1 to mediate BDNF-TrkB early endocytic trafficking and signaling from early endosomes.Mol Biol Cell. 2011 Oct;22(19):3684-98. doi: 10.1091/mbc.E11-04-0308. Epub 2011 Aug 17. Mol Biol Cell. 2011. PMID: 21849472 Free PMC article.
-
Sodium-hydrogen exchanger 6 (NHE6) deficiency leads to hearing loss, via reduced endosomal signalling through the BDNF/Trk pathway.Sci Rep. 2020 Feb 27;10(1):3609. doi: 10.1038/s41598-020-60262-5. Sci Rep. 2020. PMID: 32107410 Free PMC article.
-
Endosomal dysfunction contributes to cerebellar deficits in spinocerebellar ataxia type 6.Elife. 2023 Dec 12;12:RP90510. doi: 10.7554/eLife.90510. Elife. 2023. PMID: 38084749 Free PMC article.
-
Regulation of TrkB cell surface expression-a mechanism for modulation of neuronal responsiveness to brain-derived neurotrophic factor.Cell Tissue Res. 2020 Oct;382(1):5-14. doi: 10.1007/s00441-020-03224-7. Epub 2020 Jun 15. Cell Tissue Res. 2020. PMID: 32556728 Free PMC article. Review.
-
The Rab11-regulated endocytic pathway and BDNF/TrkB signaling: Roles in plasticity changes and neurodegenerative diseases.Neurobiol Dis. 2022 Sep;171:105796. doi: 10.1016/j.nbd.2022.105796. Epub 2022 Jun 18. Neurobiol Dis. 2022. PMID: 35728773 Review.
Cited by
-
Endophilin A1 Promotes Actin Polymerization in Dendritic Spines Required for Synaptic Potentiation.Front Mol Neurosci. 2018 May 28;11:177. doi: 10.3389/fnmol.2018.00177. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29892212 Free PMC article.
-
Loss of MUNC18-1 leads to retrograde transport defects in neurons.J Neurochem. 2021 May;157(3):450-466. doi: 10.1111/jnc.15256. Epub 2020 Dec 12. J Neurochem. 2021. PMID: 33259669 Free PMC article.
-
A triple serine motif in the intracellular domain of SorCS2 impacts its cellular signaling.iScience. 2025 May 19;28(6):112695. doi: 10.1016/j.isci.2025.112695. eCollection 2025 Jun 20. iScience. 2025. PMID: 40520096 Free PMC article.
-
PTPN23 binds the dynein adaptor BICD1 and is required for endocytic sorting of neurotrophin receptors.J Cell Sci. 2020 Mar 30;133(6):jcs242412. doi: 10.1242/jcs.242412. J Cell Sci. 2020. PMID: 32079660 Free PMC article.
-
Calcineurin and huntingtin form a calcium-sensing machinery that directs neurotrophic signals to the nucleus.Sci Adv. 2022 Jan 7;8(1):eabj8812. doi: 10.1126/sciadv.abj8812. Epub 2022 Jan 5. Sci Adv. 2022. PMID: 34985962 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

