Making Time: Conservation of Biological Clocks from Fungi to Animals
- PMID: 28527179
- PMCID: PMC5446046
- DOI: 10.1128/microbiolspec.FUNK-0039-2016
Making Time: Conservation of Biological Clocks from Fungi to Animals
Abstract
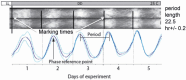
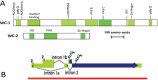
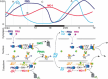
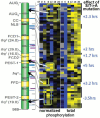
The capacity for biological timekeeping arose at least three times through evolution, in prokaryotic cyanobacteria, in cells that evolved into higher plants, and within the group of organisms that eventually became the fungi and the animals. Neurospora is a tractable model system for understanding the molecular bases of circadian rhythms in the last of these groups, and is perhaps the most intensively studied circadian cell type. Rhythmic processes described in fungi include growth rate, stress responses, developmental capacity, and sporulation, as well as much of metabolism; fungi use clocks to anticipate daily environmental changes. A negative feedback loop comprises the core of the circadian system in fungi and animals. In Neurospora, the best studied fungal model, it is driven by two transcription factors, WC-1 and WC-2, that form the White Collar Complex (WCC). WCC elicits expression of the frq gene. FRQ complexes with other proteins, physically interacts with the WCC, and reduces its activity; the kinetics of these processes is strongly influenced by progressive phosphorylation of FRQ. When FRQ becomes sufficiently phosphorylated that it loses the ability to influence WCC activity, the circadian cycle starts again. Environmental cycles of light and temperature influence frq and FRQ expression and thereby reset the internal circadian clocks. The molecular basis of circadian output is also becoming understood. Taken together, molecular explanations are emerging for all the canonical circadian properties, providing a molecular and regulatory framework that may be extended to many members of the fungal and animal kingdoms, including humans.
Figures


References
-
- Dunlap JC, Loros JJ, Decoursey P (ed). 2004. Chronobiology: Biological Timekeeping. Sinauer Assoc, Sunderland, MA.
-
- Ingold CT. 1971. Fungal Spores. Clarendon Press, Oxford, United Kingdom.
-
- Bünning E. 1973. The Physiological Clock, 3rd ed. Springer-Verlag, New York, NY. [PubMed]
-
- Brandt WH. 1953. Zonation in a prolineless strain of Neurospora. Mycologia 45:194–208.
-
- Austin B. 1968. An endogenous rhythm of spore discharge in Sordaria fimicola. Ann Bot (Lond) 32:262–278. 10.1093/oxfordjournals.aob.a084207 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

