AT1 receptor signaling pathways in the cardiovascular system
- PMID: 28527699
- PMCID: PMC5607088
- DOI: 10.1016/j.phrs.2017.05.008
AT1 receptor signaling pathways in the cardiovascular system
Abstract
The importance of the renin angiotensin aldosterone system in cardiovascular physiology and pathophysiology has been well described whereas the detailed molecular mechanisms remain elusive. The angiotensin II type 1 receptor (AT1 receptor) is one of the key players in the renin angiotensin aldosterone system. The AT1 receptor promotes various intracellular signaling pathways resulting in hypertension, endothelial dysfunction, vascular remodeling and end organ damage. Accumulating evidence shows the complex picture of AT1 receptor-mediated signaling; AT1 receptor-mediated heterotrimeric G protein-dependent signaling, transactivation of growth factor receptors, NADPH oxidase and ROS signaling, G protein-independent signaling, including the β-arrestin signals and interaction with several AT1 receptor interacting proteins. In addition, there is functional cross-talk between the AT1 receptor signaling pathway and other signaling pathways. In this review, we will summarize an up to date overview of essential AT1 receptor signaling events and their functional significances in the cardiovascular system.
Keywords: ADAM17; Angiotensin II; EGF receptor; Endothelial cell; Signal transduction; Vascular smooth muscle cell.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors are unaware of any affiliations, memberships, or financial holdings that might be perceived as affecting the objectivity of this review.
Figures
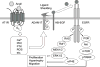
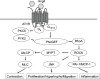
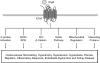
References
-
- Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2007;112(8):417–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

