Effect of Home Noninvasive Ventilation With Oxygen Therapy vs Oxygen Therapy Alone on Hospital Readmission or Death After an Acute COPD Exacerbation: A Randomized Clinical Trial
- PMID: 28528348
- PMCID: PMC5710342
- DOI: 10.1001/jama.2017.4451
Effect of Home Noninvasive Ventilation With Oxygen Therapy vs Oxygen Therapy Alone on Hospital Readmission or Death After an Acute COPD Exacerbation: A Randomized Clinical Trial
Abstract
Importance: Outcomes after exacerbations of chronic obstructive pulmonary disease (COPD) requiring acute noninvasive ventilation (NIV) are poor and there are few treatments to prevent hospital readmission and death.
Objective: To investigate the effect of home NIV plus oxygen on time to readmission or death in patients with persistent hypercapnia after an acute COPD exacerbation.
Design, setting, and participants: A randomized clinical trial of patients with persistent hypercapnia (Paco2 >53 mm Hg) 2 weeks to 4 weeks after resolution of respiratory acidemia, who were recruited from 13 UK centers between 2010 and 2015. Exclusion criteria included obesity (body mass index [BMI] >35), obstructive sleep apnea syndrome, or other causes of respiratory failure. Of 2021 patients screened, 124 were eligible.
Interventions: There were 59 patients randomized to home oxygen alone (median oxygen flow rate, 1.0 L/min [interquartile range {IQR}, 0.5-2.0 L/min]) and 57 patients to home oxygen plus home NIV (median oxygen flow rate, 1.0 L/min [IQR, 0.5-1.5 L/min]). The median home ventilator settings were an inspiratory positive airway pressure of 24 (IQR, 22-26) cm H2O, an expiratory positive airway pressure of 4 (IQR, 4-5) cm H2O, and a backup rate of 14 (IQR, 14-16) breaths/minute.
Main outcomes and measures: Time to readmission or death within 12 months adjusted for the number of previous COPD admissions, previous use of long-term oxygen, age, and BMI.
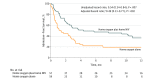
Results: A total of 116 patients (mean [SD] age of 67 [10] years, 53% female, mean BMI of 21.6 [IQR, 18.2-26.1], mean [SD] forced expiratory volume in the first second of expiration of 0.6 L [0.2 L], and mean [SD] Paco2 while breathing room air of 59 [7] mm Hg) were randomized. Sixty-four patients (28 in home oxygen alone and 36 in home oxygen plus home NIV) completed the 12-month study period. The median time to readmission or death was 4.3 months (IQR, 1.3-13.8 months) in the home oxygen plus home NIV group vs 1.4 months (IQR, 0.5-3.9 months) in the home oxygen alone group, adjusted hazard ratio of 0.49 (95% CI, 0.31-0.77; P = .002). The 12-month risk of readmission or death was 63.4% in the home oxygen plus home NIV group vs 80.4% in the home oxygen alone group, absolute risk reduction of 17.0% (95% CI, 0.1%-34.0%). At 12 months, 16 patients had died in the home oxygen plus home NIV group vs 19 in the home oxygen alone group.
Conclusions and relevance: Among patients with persistent hypercapnia following an acute exacerbation of COPD, adding home noninvasive ventilation to home oxygen therapy prolonged the time to readmission or death within 12 months.
Trial registration: clinicaltrials.gov Identifier: NCT00990132.
Conflict of interest statement
Figures

Comment in
-
Home Noninvasive Ventilation to Reduce Readmissions for Chronic Obstructive Pulmonary Disease.JAMA. 2017 Jun 6;317(21):2167-2169. doi: 10.1001/jama.2017.5226. JAMA. 2017. PMID: 28528346 No abstract available.
-
Noninvasive ventilation in hypercapnic chronic obstructive pulmonary disease.Crit Care. 2017 Oct 26;21(1):266. doi: 10.1186/s13054-017-1854-3. Crit Care. 2017. PMID: 29073934 Free PMC article. No abstract available.
-
Non-invasive positive pressure ventilation should be considered in patients with COPD and persistent hypercapnia at least 2 weeks after resolution of acute respiratory failure.Evid Based Nurs. 2018 Jan;21(1):12. doi: 10.1136/eb-2017-102789. Epub 2017 Nov 25. Evid Based Nurs. 2018. PMID: 29175966 No abstract available.
References
-
- Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817-822. - PubMed
-
- Bott J, Carroll MP, Conway JH, et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993;341(8860):1555-1557. - PubMed
-
- Connors AF Jr, Dawson NV, Thomas C, et al. ; SUPPORT Investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) . Outcomes following acute exacerbation of severe chronic obstructive lung disease. Am J Respir Crit Care Med. 1996;154(4 pt 1):959-967. - PubMed
-
- Murray I, Paterson E, Thain G, Currie GP. Outcomes following non-invasive ventilation for hypercapnic exacerbations of chronic obstructive pulmonary disease. Thorax. 2011;66(9):825-826. - PubMed

