MicroRNA Signatures and Molecular Subtypes of Glioblastoma: The Role of Extracellular Transfer
- PMID: 28528698
- PMCID: PMC5470095
- DOI: 10.1016/j.stemcr.2017.04.024
MicroRNA Signatures and Molecular Subtypes of Glioblastoma: The Role of Extracellular Transfer
Abstract
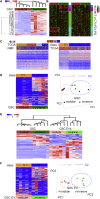
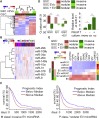
Despite the importance of molecular subtype classification of glioblastoma (GBM), the extent of extracellular vesicle (EV)-driven molecular and phenotypic reprogramming remains poorly understood. To reveal complex subpopulation dynamics within the heterogeneous intratumoral ecosystem, we characterized microRNA expression and secretion in phenotypically diverse subpopulations of patient-derived GBM stem-like cells (GSCs). As EVs and microRNAs convey information that rearranges the molecular landscape in a cell type-specific manner, we argue that intratumoral exchange of microRNA augments the heterogeneity of GSC that is reflected in highly heterogeneous profile of microRNA expression in GBM subtypes.
Keywords: GBM; cancer heterogeneity; cancer stem cells; exosomes; extracellular vesicles; glioblastoma; microRNA; subtypes.
Published by Elsevier Inc.
Figures


Similar articles
-
MicroRNA-Mediated Dynamic Bidirectional Shift between the Subclasses of Glioblastoma Stem-like Cells.Cell Rep. 2017 Jun 6;19(10):2026-2032. doi: 10.1016/j.celrep.2017.05.040. Cell Rep. 2017. PMID: 28591575 Free PMC article.
-
Extracellular vesicle-mediated transfer of CLIC1 protein is a novel mechanism for the regulation of glioblastoma growth.Oncotarget. 2015 Oct 13;6(31):31413-27. doi: 10.18632/oncotarget.5105. Oncotarget. 2015. PMID: 26429879 Free PMC article.
-
MicroRNA and extracellular vesicles in glioblastoma: small but powerful.Brain Tumor Pathol. 2016 Apr;33(2):77-88. doi: 10.1007/s10014-016-0259-3. Epub 2016 Mar 11. Brain Tumor Pathol. 2016. PMID: 26968172 Free PMC article. Review.
-
Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells.Neuro Oncol. 2012 Feb;14(2):132-44. doi: 10.1093/neuonc/nor195. Epub 2011 Nov 7. Neuro Oncol. 2012. PMID: 22067563 Free PMC article.
-
Extracellular vesicles in the biology of brain tumour stem cells--Implications for inter-cellular communication, therapy and biomarker development.Semin Cell Dev Biol. 2015 Apr;40:17-26. doi: 10.1016/j.semcdb.2015.02.011. Epub 2015 Feb 23. Semin Cell Dev Biol. 2015. PMID: 25721810 Review.
Cited by
-
MicroRNA-451 Inhibits Migration of Glioblastoma while Making It More Susceptible to Conventional Therapy.Noncoding RNA. 2019 Mar 15;5(1):25. doi: 10.3390/ncrna5010025. Noncoding RNA. 2019. PMID: 30875963 Free PMC article.
-
The importance of extracellular vesicle purification for downstream analysis: A comparison of differential centrifugation and size exclusion chromatography for helminth pathogens.PLoS Negl Trop Dis. 2019 Feb 27;13(2):e0007191. doi: 10.1371/journal.pntd.0007191. eCollection 2019 Feb. PLoS Negl Trop Dis. 2019. PMID: 30811394 Free PMC article.
-
DHHC protein family targets different subsets of glioma stem cells in specific niches.J Exp Clin Cancer Res. 2019 Jan 18;38(1):25. doi: 10.1186/s13046-019-1033-2. J Exp Clin Cancer Res. 2019. PMID: 30658672 Free PMC article.
-
Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8.J Extracell Vesicles. 2018 Mar 13;7(1):1446660. doi: 10.1080/20013078.2018.1446660. eCollection 2018. J Extracell Vesicles. 2018. PMID: 29696074 Free PMC article.
-
miR-671-5p Inhibition by MSI1 Promotes Glioblastoma Tumorigenesis via Radioresistance, Tumor Motility and Cancer Stem-like Cell Properties.Biomedicines. 2021 Dec 23;10(1):21. doi: 10.3390/biomedicines10010021. Biomedicines. 2021. PMID: 35052701 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

