Directed Differentiation of Human Pluripotent Stem Cells to Microglia
- PMID: 28528700
- PMCID: PMC5470097
- DOI: 10.1016/j.stemcr.2017.04.023
Directed Differentiation of Human Pluripotent Stem Cells to Microglia
Abstract
Microglia, the immune cells of the brain, are crucial to proper development and maintenance of the CNS, and their involvement in numerous neurological disorders is increasingly being recognized. To improve our understanding of human microglial biology, we devised a chemically defined protocol to generate human microglia from pluripotent stem cells. Myeloid progenitors expressing CD14/CX3CR1 were generated within 30 days of differentiation from both embryonic and induced pluripotent stem cells (iPSCs). Further differentiation of the progenitors resulted in ramified microglia with highly motile processes, expressing typical microglial markers. Analyses of gene expression and cytokine release showed close similarities between iPSC-derived (iPSC-MG) and human primary microglia as well as clear distinctions from macrophages. iPSC-MG were able to phagocytose and responded to ADP by producing intracellular Ca2+ transients, whereas macrophages lacked such response. The differentiation protocol was highly reproducible across several pluripotent stem cell lines.
Keywords: human microglia; human pluripotent stem cells; microglial differentiation.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

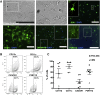
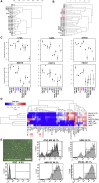

References
-
- Ajami B., Bennett J.L., Krieger C., Tetzlaff W., Rossi F.M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10:1538–1543. - PubMed
-
- Colton C.A., Gilbert D.L. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987;223:284–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

