Effects of collagen microstructure and material properties on the deformation of the neural tissues of the lamina cribrosa
- PMID: 28528864
- PMCID: PMC5537032
- DOI: 10.1016/j.actbio.2017.05.042
Effects of collagen microstructure and material properties on the deformation of the neural tissues of the lamina cribrosa
Abstract
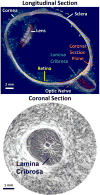
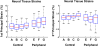
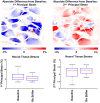
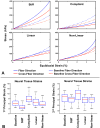
It is widely considered that intraocular pressure (IOP)-induced deformation within the neural tissue pores of the lamina cribrosa (LC) contributes to neurodegeneration and glaucoma. Our goal was to study how the LC microstructure and mechanical properties determine the mechanical insult to the neural tissues within the pores of the LC. Polarized light microscopy was used to measure the collagen density and orientation in histology sections of three sheep optic nerve heads (ONH) at both mesoscale (4.4μm) and microscale (0.73μm) resolutions. Mesoscale fiber-aware FE models were first used to calculate ONH deformations at an IOP of 30mmHg. The results were then used as boundary conditions for microscale models of LC regions. Models predicted large insult to the LC neural tissues, with 95th percentile 1st principal strains ranging from 7 to 12%. Pores near the scleral boundary suffered significantly higher stretch compared to pores in more central regions (10.0±1.4% vs. 7.2±0.4%; p=0.014; mean±SD). Variations in material properties altered the minimum, median, and maximum levels of neural tissue insult but largely did not alter the patterns of pore-to-pore variation, suggesting these patterns are determined by the underlying structure and geometry of the LC beams and pores. To the best of our knowledge, this is the first computational model that reproduces the highly heterogeneous neural tissue strain fields observed experimentally.
Statement of significance: The loss of visual function associated with glaucoma has been attributed to sustained mechanical insult to the neural tissues of the lamina cribrosa due to elevated intraocular pressure. Our study is the first computational model built from specimen-specific tissue microstructure to consider the mechanics of the neural tissues of the lamina separately from the connective tissue. We found that the deformation of the neural tissue was much larger than that predicted by any recent microstructure-aware models of the lamina. These results are consistent with recent experimental data and the highest deformations were found in the region of the lamina where glaucomatous damage first occurs. This study provides new insight into the complex biomechanical environment within the lamina.
Keywords: Biomechanics; Finite element modeling; Glaucoma; Intraocular pressure; Lamina cribrosa; Microstructure; Optic nerve head.
Copyright © 2017 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures






References
-
- Hernandez MR. The optic nerve head in glaucoma: Role of astrocytes in tissue remodeling. Progress in Retinal and Eye Research. 2000;19:297–321. - PubMed
-
- Sigal IA, Ethier CR. Biomechanics of the optic nerve head. Experimental eye research. 2009:799–807. - PubMed
-
- Quigley HA. Reappraisal of the mechanisms of glaucomatous optic nerve damage. Eye (London, England) 1987;1(Pt 2):318–22. - PubMed
-
- Bengtsson B, Heijl A. A long-term prospective study of risk factors for glaucomatous visual field loss in patients with ocular hypertension. J Glaucoma. 2005;14:135–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

