Fragile X-Associated Tremor/Ataxia Syndrome: From Molecular Pathogenesis to Development of Therapeutics
- PMID: 28529475
- PMCID: PMC5418347
- DOI: 10.3389/fncel.2017.00128
Fragile X-Associated Tremor/Ataxia Syndrome: From Molecular Pathogenesis to Development of Therapeutics
Abstract
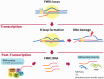
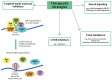
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a neurodegenerative disorder caused by a premutation CGG repeat expansion (55-200 repeats) within the 5' UTR of the fragile X gene (FMR1). FXTAS is characterized by intension tremor, cerebellar ataxia, progressive neurodegeneration, parkinsonism and cognitive decline. The development of transgenic mouse and Drosophila melanogaster models carrying an expanded CGG repeat has yielded valuable insight into the pathophysiology of FXTAS. To date, we know of two main molecular mechanisms of this disorder: (1) a toxic gain of function of the expanded CGG-repeat FMR1 mRNA, which results in the binding/sequestration of the CGG-binding proteins; and (2) CGG repeat-associated non-AUG-initiated (RAN) translation, which generates a polyglycine peptide toxic to cells. Besides these CGG-mediated mechanisms, recent studies have shed light on additional mechanisms of pathogenesis, such as the antisense transcript ASFMR1, mitochondrial dysfunction, DNA damage from R-loop formation and 5-hydroxymethylcytosine (5hmC)-mediated epigenetic modulation. Here we summarize the recent progress towards understanding the etiology of FXTAS and provide an overview of potential treatment strategies.
Keywords: CGG repeat; FXTAS pathogenesis; FXTAS therapeutics; RAN translation; RNA toxicity.
Figures
Similar articles
-
RAN translation at CGG repeats induces ubiquitin proteasome system impairment in models of fragile X-associated tremor ataxia syndrome.Hum Mol Genet. 2015 Aug 1;24(15):4317-26. doi: 10.1093/hmg/ddv165. Epub 2015 May 7. Hum Mol Genet. 2015. PMID: 25954027 Free PMC article.
-
Repeat-associated non-AUG (RAN) translation and other molecular mechanisms in Fragile X Tremor Ataxia Syndrome.Brain Res. 2018 Aug 15;1693(Pt A):43-54. doi: 10.1016/j.brainres.2018.02.006. Epub 2018 Feb 14. Brain Res. 2018. PMID: 29453961 Free PMC article. Review.
-
Repeat-associated non-AUG translation from antisense CCG repeats in fragile X tremor/ataxia syndrome.Ann Neurol. 2016 Dec;80(6):871-881. doi: 10.1002/ana.24800. Epub 2016 Nov 26. Ann Neurol. 2016. PMID: 27761921 Free PMC article.
-
Piperine Modulates Protein Mediated Toxicity in Fragile X-Associated Tremor/Ataxia Syndrome through Interacting Expanded CGG Repeat (r(CGG)exp) RNA.ACS Chem Neurosci. 2019 Aug 21;10(8):3778-3788. doi: 10.1021/acschemneuro.9b00282. Epub 2019 Jul 2. ACS Chem Neurosci. 2019. PMID: 31264835
-
Molecular Pathophysiology of Fragile X-Associated Tremor/Ataxia Syndrome and Perspectives for Drug Development.Cerebellum. 2016 Oct;15(5):599-610. doi: 10.1007/s12311-016-0800-2. Cerebellum. 2016. PMID: 27277287 Review.
Cited by
-
Identification of PSMB5 as a genetic modifier of fragile X-associated tremor/ataxia syndrome.Proc Natl Acad Sci U S A. 2022 May 31;119(22):e2118124119. doi: 10.1073/pnas.2118124119. Epub 2022 May 26. Proc Natl Acad Sci U S A. 2022. PMID: 35617426 Free PMC article.
-
The Application of Adeno-Associated Viral Vector Gene Therapy to the Treatment of Fragile X Syndrome.Brain Sci. 2019 Feb 2;9(2):32. doi: 10.3390/brainsci9020032. Brain Sci. 2019. PMID: 30717399 Free PMC article. Review.
-
Repeat-associated non-ATG (RAN) translation.J Biol Chem. 2018 Oct 19;293(42):16127-16141. doi: 10.1074/jbc.R118.003237. Epub 2018 Sep 13. J Biol Chem. 2018. PMID: 30213863 Free PMC article.
-
Prodromal Markers of Upper Limb Deficits in FMR1 Premutation Carriers and Quantitative Outcome Measures for Future Clinical Trials in Fragile X-associated Tremor/Ataxia Syndrome.Mov Disord Clin Pract. 2020 Aug 29;7(7):810-819. doi: 10.1002/mdc3.13045. eCollection 2020 Oct. Mov Disord Clin Pract. 2020. PMID: 33043077 Free PMC article.
-
Metabolic pathways modulate the neuronal toxicity associated with fragile X-associated tremor/ataxia syndrome.Hum Mol Genet. 2019 Mar 15;28(6):980-991. doi: 10.1093/hmg/ddy410. Hum Mol Genet. 2019. PMID: 30476102 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

