Hormonoresistance in advanced breast cancer: a new revolution in endocrine therapy
- PMID: 28529550
- PMCID: PMC5424863
- DOI: 10.1177/1758834017693195
Hormonoresistance in advanced breast cancer: a new revolution in endocrine therapy
Abstract
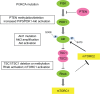
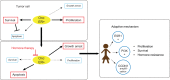
Endocrine therapy is the mainstay of treatment of estrogen-receptor-positive (ER+) breast cancer with an overall survival benefit. However, some adaptive mechanisms in the tumor emerge leading to the development of a resistance to this therapy. A better characterization of this process is needed to overcome this resistance and to develop new tailored therapies. Mechanisms of resistance to hormone therapy result in activation of transduction signal pathways, including the cell cycle regulation with cyclin D/CDK4/6/Rb pathway. The strategy of combined hormone therapy with targeted agents has shown an improvement of progression-free survival (PFS) in several phase II or III trials, including three different classes of drugs: mTOR inhibitors, PI3K and CDK4/6 inhibitors. A recent phase III trial has shown that fulvestrant combined with a CDK 4/6 inhibitor doubles PFS in aromatase inhibitor-pretreated postmenopausal ER+ breast cancer. Other combinations are ongoing to disrupt the interaction between PI3K/AKT/mTOR and cyclin D/CDK4/6/Rb pathways. Despite these successful strategies, reliable and reproducible biomarkers are needed. Tumor genomics are dynamic over time, and blood-based biomarkers such as circulating tumor DNA represent a major hope to elucidate the adaptive mechanisms of endocrine resistance. The optimal combinations and biomarkers to guide this strategy need to be determined.
Keywords: CDK4/6 inhibitor; PI3K/mTOR inhibitor; adaptive mechanism; hormonoresistance.
Conflict of interest statement
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 2014; 23: 489–502. - PubMed
-
- Johnston SR. New strategies in estrogen receptor-positive breast cancer. Clin Cancer Res 2010; 16: 1979–1987. - PubMed
-
- Tryfonidis K, Basaran G, Bogaerts J, et al. A European Organisation for Research and Treatment of Cancer randomized, double-blind, placebo-controlled, multicentre phase II trial of anastrozole in combination with gefitinib or placebo in hormone receptor-positive advanced breast cancer [ClinicalTrials.gov identifier: NCT00066378]. Eur J Cancer 2016; 53:144–154. - PubMed
-
- Robertson JF, Ferrero JM, Bourgeois H, et al. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol 2013;14: 228–235. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous