A single-center experience of sorafenib monotherapy in patients with advanced intrahepatic cholangiocarcinoma
- PMID: 28529557
- PMCID: PMC5431743
- DOI: 10.3892/ol.2017.5847
A single-center experience of sorafenib monotherapy in patients with advanced intrahepatic cholangiocarcinoma
Abstract
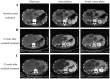
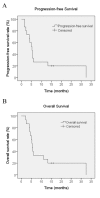
Patients with advanced intrahepatic cholangiocarcinoma (ICC) have a poor prognosis and the therapeutic options available for treating ICC are limited. Sorafenib, a multikinase inhibitor of vascular endothelial growth factor receptor 2 and 3, platelet derived growth factor receptor-β, B-Raf proto-oncogene, serine/threonine kinase and C-Raf proto-oncogene, serine/threonine kinase, is a novel reference standard for the treatment of advanced hepatocellular carcinoma. Sorafenib has previously been demonstrated to exhibit significant antitumor activity in various cholangiocarcinoma cell lines and in xenograft ICC models. The present study aimed to assess the efficacy and safety of sorafenib as a single-agent treatment in patients with advanced ICC. Eligible patients were administere no prior therapy for metastatic or unresectable disease. Sorafenib was administered orally at a dose of 400 mg twice daily continuously. The primary endpoint was considered as the disease control rate (DCR) at 12 weeks. Secondary endpoints included time to progression (TTP), progression-free survival (PFS), overall survival (OS), duration of treatment (DOT) and the adverse event profile. A total of 15 patients were enrolled in the present study, with a median DOT of 3.2 months (range, 1.5-30 months). A total of 4 patients achieved a partial response and 7 patients achieved stable disease, with a DCR of 73.3%. The median OS time was 5.7 months [95% confidence interval (CI), 5.0-6.4], the PFS time was 5.5 months (95% CI, 3.9-7.1) and the median TTP was 3.2 months (range, 1.5-29 months). The most common toxicity was a skin rash, which w1as observed in 5 patients (33.3%). Grade 3 hand-foot syndrome was observed in 1 patient (6.7%), which required treatment termination. The results of the present study suggest that sorafenib monotherapy may exhibit promising anticancer activity in patients with advanced ICC and that it has a manageable toxicity profile.
Keywords: advanced; intrahepatic cholangiocarcinoma; sorafenib; toxicity.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
