Allograft Bone as Antibiotic Carrier
- PMID: 28529864
- PMCID: PMC5423575
- DOI: 10.7150/jbji.17466
Allograft Bone as Antibiotic Carrier
Abstract
The treatment of chronic bone and joint infections is characterized by obstinate persistency of the causing microorganisms and resulting long term disability to patients, associated with remarkable costs for the health care system. Difficulties derive from biofilm formed on dead bone and eventual implants, with resistance against immunological defences and antimicrobial substances. Biofilm embedded bacteria require up to 1000 times the antibiotic concentration of planktonic bacteria for elimination. Systemic antibiotic treatment alone cannot provide the concentrations required and surgical intervention is always prerequisite for potentially providing a cure. A second issue is that osseous defects are almost always present after surgical debridement, and it is difficult to address their reconstruction. One option is to use bone grafts, either from the patient´s own body or from foreign donors (allografts). Grafts are usually unvascularized and are prone to colonization with bacteria. Loading of allografts with antibiotics may not only protect grafts from bacterial adhesion but, using appropriate processing methods, may also provide high local antibiotic concentrations that may eliminate remaining sessile pathogens. For efficient action as antibiotic carriers, the release of antibiotics should be above the minimum biofilm eradication concentration (MBEC) for a prolonged period of time. Cleaning the bone from bone marrow opens a large reservoir for storage of antimicrobial substances that, after implantation, may be released to the surrounding in a sustained mode, possibly eliminating remaining biofilm remnants. Removal of bone marrow, leaving a pure matrix, provides increased safety and improved revascularization of the graft. Local provision of antibiotic concentrations above the MBEC may enable simultaneous internal fixation with osteosynthetic material and single stage exchange of infected endoprostheses, resulting in shorter hospital stays with reduced pain and faster rehabilitation of patients.
Keywords: allograft; antibiotic carrier; biofilm; bone; infection; one stage treatment; quality of life.; reconstruction.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






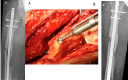

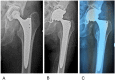
References
-
- Costerton JW. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin Orthop Relat Res. 2005:7–11. - PubMed
-
- Lichstein P, Gehrke T, Lombardi A. et al. One-stage vs two-stage exchange. J Arthroplasty. 2014;29:108–11. - PubMed
-
- Masquelet AC, Fitoussi F, Begue T, Muller GP. [Reconstruction of the long bones by the induced membrane and spongy autograft] Ann Chir Plast Esthet. 2000;45:346–53. - PubMed
-
- Karger C, Kishi T, Schneider L, Fitoussi F, Masquelet AC. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop Traumatol Surg Res. 2012;98:97–102. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources