Neuroimaging in pre-motor Parkinson's disease
- PMID: 28529878
- PMCID: PMC5429242
- DOI: 10.1016/j.nicl.2017.04.011
Neuroimaging in pre-motor Parkinson's disease
Abstract
The process of neurodegeneration in Parkinson's disease begins long before the onset of clinical motor symptoms, resulting in substantial cell loss by the time a diagnosis can be made. The period between the onset of neurodegeneration and the development of motoric disease would be the ideal time to intervene with disease modifying therapies. This pre-motor phase can last many years, but the lack of a specific clinical phenotype means that objective biomarkers are needed to reliably detect prodromal disease. In recent years, recognition that patients with REM sleep behaviour disorder (RBD) are at particularly high risk of future parkinsonism has enabled the development of large prodromal cohorts in which to investigate novel biomarkers, and neuroimaging has generated some of the most promising results to date. Here we review investigations undertaken in RBD and other pre-clinical cohorts, including modalities that are well established in clinical Parkinson's as well as novel imaging methods. Techniques such as high resolution MRI of the substantia nigra and functional imaging of Parkinsonian brain networks have great potential to facilitate early diagnosis. Further longitudinal studies will establish their true value in quantifying prodromal neurodegeneration and predicting future Parkinson's.
Figures

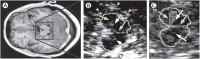

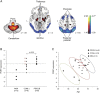

References
-
- Adams J.R., van Netten H., Schulzer M. PET in LRRK2 mutations: comparison to sporadic Parkinson's disease and evidence for presymptomatic compensation. Brain. 2005;128:2777–2785. - PubMed
-
- Albin R.L., Koeppe R.A., Chervin R.D., Consens F.B., Wernette K., Frey K.A. Decreased striatal dopaminergic innervation in REM sleep behavior disorder. Neurology. 2000;55(9):1410–1412. - PubMed
-
- Arnaldi D., de Carli F., Picco A., Ferrara M., Accardo J., Bossert I. Nigro-caudate dopaminergic deafferentation: a marker of REM sleep behavior disorder? Neurobiol. Aging. 2015;36(12):3300–3305. - PubMed
-
- Barkhof F., Haller S., Rombouts S.A. Resting-state functional MR imaging: a new window to the brain. Radiology. 2014 Jul;272(1):29–49. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

