Manipulation of Host Cholesterol by Obligate Intracellular Bacteria
- PMID: 28529926
- PMCID: PMC5418226
- DOI: 10.3389/fcimb.2017.00165
Manipulation of Host Cholesterol by Obligate Intracellular Bacteria
Abstract
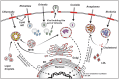
Cholesterol is a multifunctional lipid that plays important metabolic and structural roles in the eukaryotic cell. Despite having diverse lifestyles, the obligate intracellular bacterial pathogens Chlamydia, Coxiella, Anaplasma, Ehrlichia, and Rickettsia all target cholesterol during host cell colonization as a potential source of membrane, as well as a means to manipulate host cell signaling and trafficking. To promote host cell entry, these pathogens utilize cholesterol-rich microdomains known as lipid rafts, which serve as organizational and functional platforms for host signaling pathways involved in phagocytosis. Once a pathogen gains entrance to the intracellular space, it can manipulate host cholesterol trafficking pathways to access nutrient-rich vesicles or acquire membrane components for the bacteria or bacteria-containing vacuole. To acquire cholesterol, these pathogens specifically target host cholesterol metabolism, uptake, efflux, and storage. In this review, we examine the strategies obligate intracellular bacterial pathogens employ to manipulate cholesterol during host cell colonization. Understanding how obligate intracellular pathogens target and use host cholesterol provides critical insight into the host-pathogen relationship.
Keywords: Anaplasma; Chlamydia; Coxiella; Rickettsia; cholesterol; lipid droplet; lipid raft.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

