Complement C5a Receptor is the Key Initiator of Neutrophil Adhesion Igniting Immune Complex-induced Arthritis
- PMID: 28529998
- PMCID: PMC5436313
- DOI: 10.1126/sciimmunol.aaj2195
Complement C5a Receptor is the Key Initiator of Neutrophil Adhesion Igniting Immune Complex-induced Arthritis
Abstract
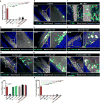
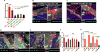
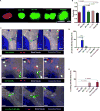
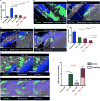
The deposition of immune complexes (IC) in tissues induces a "type III hypersensitivity" that results in tissue damage and underlies the pathogenesis of many autoimmune diseases. The neutrophil is the first immune cell recruited into sites of IC deposition and plays a critical role in shaping the overall tissue response. However, the mechanism by which IC initiate and propagate neutrophil infiltration into tissue is not known. Here, using intravital multiphoton joint imaging of IC-induced arthritis in live mice, we found that the complement C5a receptor (C5aR) was the key initiator of neutrophil adhesion on joint endothelium. C5a presented on joint endothelium induced β2 integrin-dependent neutrophil arrest, facilitating neutrophil spreading and transition to crawling, and subsequent leukotriene B4 receptor (BLT1)-mediated extravasation of the first neutrophils. The chemokine receptor CCR1 promoted neutrophil crawling on the joint endothelium while CXCR2 amplified late neutrophil recruitment and survival once in the joint. Thus, imaging arthritis has defined a new paradigm for type III hypersensitivity where C5a directly initiates neutrophil adhesion on the joint endothelium igniting inflammation.
Conflict of interest statement
Competing interests: The authors declare no competing financial interests.
Figures




Similar articles
-
The critical role of C5a as an initiator of neutrophil-mediated autoimmune inflammation of the joint and skin.Semin Immunol. 2018 Jun;37:21-29. doi: 10.1016/j.smim.2018.03.002. Epub 2018 Mar 27. Semin Immunol. 2018. PMID: 29602515 Review.
-
Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcγR signaling.Proc Natl Acad Sci U S A. 2012 Nov 13;109(46):E3177-85. doi: 10.1073/pnas.1213797109. Epub 2012 Oct 29. Proc Natl Acad Sci U S A. 2012. PMID: 23112187 Free PMC article.
-
Atypical complement receptor C5aR2 transports C5a to initiate neutrophil adhesion and inflammation.Sci Immunol. 2019 May 10;4(35):eaav5951. doi: 10.1126/sciimmunol.aav5951. Sci Immunol. 2019. PMID: 31076525
-
Treatment with anti-C5aR mAb leads to early-onset clinical and mechanistic effects in the murine delayed-type hypersensitivity arthritis model.Autoimmunity. 2015;48(7):460-70. doi: 10.3109/08916934.2015.1031888. Epub 2015 Apr 27. Autoimmunity. 2015. PMID: 25915570
-
Role of C5a-C5aR interaction in sepsis.Shock. 2004 Jan;21(1):1-7. doi: 10.1097/01.shk.0000105502.75189.5e. Shock. 2004. PMID: 14676676 Review.
Cited by
-
Synergistic antitumor activity by dual blockade of CCR1 and CXCR2 expressed on myeloid cells within the tumor microenvironment.Br J Cancer. 2024 Jul;131(1):63-76. doi: 10.1038/s41416-024-02710-x. Epub 2024 May 15. Br J Cancer. 2024. PMID: 38750114 Free PMC article.
-
Eculizumab treatment alters the proteometabolome beyond the inhibition of complement.JCI Insight. 2023 Jul 10;8(13):e169135. doi: 10.1172/jci.insight.169135. JCI Insight. 2023. PMID: 37227781 Free PMC article.
-
Marker Genes Change of Synovial Fibroblasts in Rheumatoid Arthritis Patients.Biomed Res Int. 2021 Jun 4;2021:5544264. doi: 10.1155/2021/5544264. eCollection 2021. Biomed Res Int. 2021. PMID: 34195267 Free PMC article.
-
Renal diseases and the role of complement: Linking complement to immune effector pathways and therapeutics.Adv Immunol. 2021;152:1-81. doi: 10.1016/bs.ai.2021.09.001. Epub 2021 Nov 19. Adv Immunol. 2021. PMID: 34844708 Free PMC article. Review.
-
Neutrophils in the periodontium: Interactions with pathogens and roles in tissue homeostasis and inflammation.Immunol Rev. 2023 Mar;314(1):93-110. doi: 10.1111/imr.13152. Epub 2022 Oct 22. Immunol Rev. 2023. PMID: 36271881 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

