The E368Q Mutant Allele of GJA8 is Associated with Congenital Cataracts with Intrafamilial Variation in a South Indian Family
- PMID: 28530003
- PMCID: PMC5438206
The E368Q Mutant Allele of GJA8 is Associated with Congenital Cataracts with Intrafamilial Variation in a South Indian Family
Abstract
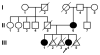
Purpose: To determine the basis of the autosomal dominant congenital cataracts in a three generation south Indian pedigree.
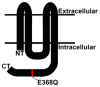
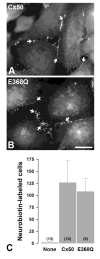
Methods: The proband and several family members underwent a complete ophthalmic examination. The coding regions of eight candidate genes (CRYAA, CRYBB2, CRYGC, CRYGD, GJA3, GJA8, AQP0, and PITX3) were amplified by PCR and directly sequenced. Wild type and mutant connexin50 (Cx50) were expressed by stable transfection of HeLa cells. Their cellular distributions and function were examined by immunofluorescence microscopy and by microinjection of gap junction permeant tracers, respectively.
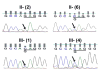
Results: Congenital cataracts (with some variations in phenotype) segregated as an autosomal dominant trait within a three generation pedigree. Three affected individuals (proband, sibling and mother) showed a sequence variation in the candidate gene GJA8 encoding Cx50: a c.1102G>C transversion encoding a substitution of glutamate for glutamine at position 368 (E368Q). This substitution was absent from an unaffected family member (paternal aunt) and 100 healthy controls of the same ethnicity. In transfected HeLa cells, both wild type Cx50 and E368Q localized to gap junction plaques, and supported similar levels of intercellular transfer of Neurobiotin.
Conclusions: The E368Q mutant allele of GJA8 is associated with autosomal dominant congenital cataracts with phenotypic variability. E368Q forms gap junction plaques and functional channels in transfected HeLa cells.
Figures
Similar articles
-
A novel connexin50 mutation associated with congenital nuclear pulverulent cataracts.J Med Genet. 2008 Mar;45(3):155-60. doi: 10.1136/jmg.2007.051029. Epub 2007 Nov 15. J Med Genet. 2008. PMID: 18006672 Free PMC article.
-
Characterization of a variant of gap junction protein α8 identified in a family with hereditary cataract.PLoS One. 2017 Aug 21;12(8):e0183438. doi: 10.1371/journal.pone.0183438. eCollection 2017. PLoS One. 2017. PMID: 28827829 Free PMC article.
-
The GJA8 allele encoding CX50I247M is a rare polymorphism, not a cataract-causing mutation.Mol Vis. 2009 Sep 14;15:1881-5. Mol Vis. 2009. PMID: 19756179 Free PMC article.
-
Cataracts are caused by alterations of a critical N-terminal positive charge in connexin50.Invest Ophthalmol Vis Sci. 2008 Jun;49(6):2549-56. doi: 10.1167/iovs.07-1658. Epub 2008 Mar 7. Invest Ophthalmol Vis Sci. 2008. PMID: 18326694 Free PMC article.
-
[Progress in pathogenic genes and their functions of congenital cataract].Zhonghua Yan Ke Za Zhi. 2010 Mar;46(3):280-4. Zhonghua Yan Ke Za Zhi. 2010. PMID: 20450675 Review. Chinese.
Cited by
-
Identification of a New Mutation p.P88L in Connexin 50 Associated with Dominant Congenital Cataract.Front Cell Dev Biol. 2022 Apr 21;10:794837. doi: 10.3389/fcell.2022.794837. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35531093 Free PMC article.
References
-
- World Health Organization. Prevention of Childhood Blindness. WHO; 1992.
-
- Rahi JS, Dezateux C. Measuring and interpreting the incidence of congenital ocular anomalies: lessons from a national study of congenital cataract in the UK. Invest Ophthalmol Vis Sci. 2001;42(7):1444–1448. - PubMed
-
- Gilbert CE, Canovas R, Hagan M, Rao S, Foster A. Causes of childhood blindness: results from west Africa, south India and Chile. Eye (Lond) 1993;7(1):184–188. - PubMed
-
- Dandona L, Williams JD, Williams BC, Rao GN. Population-based assessment of childhood blindness in southern India. Arch Ophthalmol. 1998;116(4):545–546. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
