Calcium-binding protein S100A4 confers mesenchymal progenitor cell fibrogenicity in idiopathic pulmonary fibrosis
- PMID: 28530639
- PMCID: PMC5490771
- DOI: 10.1172/JCI90832
Calcium-binding protein S100A4 confers mesenchymal progenitor cell fibrogenicity in idiopathic pulmonary fibrosis
Abstract
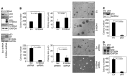
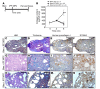
Idiopathic pulmonary fibrosis (IPF) is a progressive disease with a prevalence of 1 million persons worldwide. The fibrosis spreads from affected alveoli into contiguous alveoli and leads to death by asphyxiation. We previously discovered that the IPF lung harbors fibrogenic mesenchymal progenitor cells (MPCs) that serve as a cell of origin for disease-mediating myofibroblasts. In a prior genomewide transcriptional analysis, we found that IPF MPCs displayed increased expression of S100 calcium-binding A4 (S100A4), a protein linked to cancer cell proliferation and invasiveness. Here, we have examined whether S100A4 mediates MPC fibrogenicity. Ex vivo analysis revealed that IPF MPCs had increased levels of nuclear S100A4, which interacts with L-isoaspartyl methyltransferase to promote p53 degradation and MPC self-renewal. In vivo, injection of human IPF MPCs converted a self-limited bleomycin-induced mouse model of lung fibrosis to a model of persistent fibrosis in an S100A4-dependent manner. S100A4 gain of function was sufficient to confer fibrotic properties to non-IPF MPCs. In IPF tissue, fibroblastic foci contained cells expressing Ki67 and the MPC markers SSEA4 and S100A4. The expression colocalized in an interface region between myofibroblasts in the focus core and normal alveolar structures, defining this region as an active fibrotic front. Our findings indicate that IPF MPCs are intrinsically fibrogenic and that S100A4 confers MPCs with fibrogenicity.
Conflict of interest statement
Figures




References
-
- Noble PW. Idiopathic pulmonary fibrosis: natural history and prognosis. Clin Chest Med. 2006;27(1 Suppl 1):S11–S16. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

