MYC-dependent oxidative metabolism regulates osteoclastogenesis via nuclear receptor ERRα
- PMID: 28530645
- PMCID: PMC5490751
- DOI: 10.1172/JCI89935
MYC-dependent oxidative metabolism regulates osteoclastogenesis via nuclear receptor ERRα
Abstract
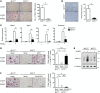
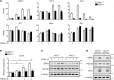
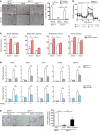
Osteoporosis is a metabolic bone disorder associated with compromised bone strength and an increased risk of fracture. Inhibition of the differentiation of bone-resorbing osteoclasts is an effective strategy for the treatment of osteoporosis. Prior work by our laboratory and others has shown that MYC promotes osteoclastogenesis in vitro, but the underlying mechanisms are not well understood. In addition, the in vivo importance of osteoclast-expressed MYC in physiological and pathological bone loss is not known. Here, we have demonstrated that deletion of Myc in osteoclasts increases bone mass and protects mice from ovariectomy-induced (OVX-induced) osteoporosis. Transcriptomic analysis revealed that MYC drives metabolic reprogramming during osteoclast differentiation and functions as a metabolic switch to an oxidative state. We identified a role for MYC action in the transcriptional induction of estrogen receptor-related receptor α (ERRα), a nuclear receptor that cooperates with the transcription factor nuclear factor of activated T cells, c1 (NFATc1) to drive osteoclastogenesis. Accordingly, pharmacological inhibition of ERRα attenuated OVX-induced bone loss in mice. Our findings highlight a MYC/ERRα pathway that contributes to physiological and pathological bone loss by integrating the MYC/ERRα axis to drive metabolic reprogramming during osteoclast differentiation.
Conflict of interest statement
Figures




Comment in
- The many ways of osteoclast activation doi: 10.1172/JCI94606
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

